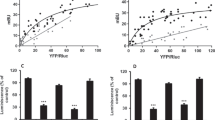
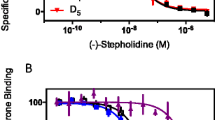
Abstract
The poor norepinephrine innervation and high density of Gi/o-coupled α2A- and α2C-adrenoceptors in the striatum and the dense striatal dopamine innervation have prompted the possibility that dopamine could be an effective adrenoceptor ligand. Nevertheless, the reported adrenoceptor agonistic properties of dopamine are still inconclusive. In this study, we analyzed the binding of norepinephrine, dopamine, and several compounds reported as selective dopamine D2-like receptor ligands, such as the D3 receptor agonist 7-OH-PIPAT and the D4 receptor agonist RO-105824, to α2-adrenoceptors in cortical and striatal tissue, which express α2A-adrenoceptors and both α2A- and α2C-adrenoceptors, respectively. The affinity of dopamine for α2-adrenoceptors was found to be similar to that for D1-like and D2-like receptors. Moreover, the exogenous dopamine receptor ligands also showed high affinity for α2A- and α2C-adrenoceptors. Their ability to activate Gi/o proteins through α2A- and α2C-adrenoceptors was also analyzed in transfected cells with bioluminescent resonance energy transfer techniques. The relative ligand potencies and efficacies were dependent on the Gi/o protein subtype. Furthermore, dopamine binding to α2-adrenoceptors was functional, inducing changes in dynamic mass redistribution, adenylyl cyclase activity, and ERK1/2 phosphorylation. Binding events were further studied with computer modeling of ligand docking. Docking of dopamine at α2A- and α2C-adrenoceptors was nearly identical to its binding to the crystallized D3 receptor. Therefore, we provide conclusive evidence that α2A- and α2C-adrenoceptors are functional receptors for norepinephrine, dopamine, and other previously assumed selective D2-like receptor ligands, which calls for revisiting previous studies with those ligands.









Similar content being viewed by others
References
Alexander SP, Mathie A, Peters JA (2011) Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol 164(Suppl 1):S1–S324. https://doi.org/10.1111/j.1476-5381.2011.01649_1.x
MacDonald E, Kobilka BK, Scheinin M (1997) Gene targeting—homing in on alpha 2-adrenoceptor-subtype function. Trends Pharmacol Sci 18(6):211–219
Nicholas AP, Pieribone V, Hokfelt T (1993) Distributions of mRNAs for alpha-2 adrenergic receptor subtypes in rat brain: an in situ hybridization study. J Comp Neurol 328(4):575–594. https://doi.org/10.1002/cne.903280409
Scheinin M, Lomasney JW, Hayden-Hixson DM, Schambra UB, Caron MG, Lefkowitz RJ, Fremeau RT Jr (1994) Distribution of alpha 2-adrenergic receptor subtype gene expression in rat brain. Brain Res Mol Brain Res 21(1–2):133–149
Fagerholm V, Rokka J, Nyman L, Sallinen J, Tiihonen J, Tupala E, Haaparanta M, Hietala J (2008) Autoradiographic characterization of alpha(2C)-adrenoceptors in the human striatum. Synapse 62(7):508–515. https://doi.org/10.1002/syn.20520
Lehto J, Virta JR, Oikonen V, Roivainen A, Luoto P, Arponen E, Helin S, Hietamaki J et al (2015) Test-retest reliability of (11)C-ORM-13070 in PET imaging of alpha2C-adrenoceptors in vivo in the human brain. Eur J Nucl Med Mol Imaging 42(1):120–127. https://doi.org/10.1007/s00259-014-2899-z
Ordway GA, Jaconetta SM, Halaris AE (1993) Characterization of subtypes of alpha-2 adrenoceptors in the human brain. J Pharmacol Exp Ther 264(2):967–976
Uhlen S, Lindblom J, Tiger G, Wikberg JE (1997) Quantification of alpha2A and alpha2C adrenoceptors in the rat striatum and in different regions of the spinal cord. Acta Physiol Scand 160(4):407–412. https://doi.org/10.1046/j.1365-201X.1997.00175.x
Lindvall O, Bjorklund A (1974) The organization of the ascending catecholamine neuron systems in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta Physiol Scand Suppl 412:1–48
Swanson LW, Hartman BK (1975) The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-beta-hydroxylase as a marker. J Comp Neurol 163(4):467–505. https://doi.org/10.1002/cne.901630406
Aston-Jones G (2004) Locus coeruleus, A5 and A7 noradrenergic cell groups. In: Paxinos G (ed) The rat nervous system. Academic, San Diego, pp. 259–294
Gobert A, Billiras R, Cistarelli L, Millan MJ (2004) Quantification and pharmacological characterization of dialysate levels of noradrenaline in the striatum of freely-moving rats: release from adrenergic terminals and modulation by alpha2-autoreceptors. J Neurosci Methods 140(1–2):141–152. https://doi.org/10.1016/j.jneumeth.2004.04.040
Holmberg M, Scheinin M, Kurose H, Miettinen R (1999) Adrenergic alpha2C-receptors reside in rat striatal GABAergic projection neurons: comparison of radioligand binding and immunohistochemistry. Neuroscience 93(4):1323–1333
Hara M, Fukui R, Hieda E, Kuroiwa M, Bateup HS, Kano T, Greengard P, Nishi A (2010) Role of adrenoceptors in the regulation of dopamine/DARPP-32 signaling in neostriatal neurons. J Neurochem 113(4):1046–1059. https://doi.org/10.1111/j.1471-4159.2010.06668.x
Ihalainen JA, Tanila H (2004) In vivo regulation of dopamine and noradrenaline release by alpha2A-adrenoceptors in the mouse nucleus accumbens. J Neurochem 91(1):49–56. https://doi.org/10.1111/j.1471-4159.2004.02691.x
Zhang W, Klimek V, Farley JT, Zhu MY, Ordway GA (1999) alpha2C adrenoceptors inhibit adenylyl cyclase in mouse striatum: potential activation by dopamine. J Pharmacol Exp Ther 289(3):1286–1292
Alachkar A, Brotchie JM, Jones OT (2010) Binding of dopamine and 3-methoxytyramine as l-DOPA metabolites to human alpha(2)-adrenergic and dopaminergic receptors. Neurosci Res 67(3):245–249. https://doi.org/10.1016/j.neures.2010.03.008
Cornil CA, Ball GF (2008) Interplay among catecholamine systems: dopamine binds to alpha2-adrenergic receptors in birds and mammals. J Comp Neurol 511(5):610–627. https://doi.org/10.1002/cne.21861
Sánchez-Soto M, Bonifazi A, Cai NS, Ellenberger MP, Newman AH, Ferré S, Yano H (2016) Evidence for noncanonical neurotransmitter activation: norepinephrine as a dopamine D2-like receptor agonist. Mol Pharmacol 89(4):457–466. https://doi.org/10.1124/mol.115.101808
Urizar E, Yano H, Kolster R, Gales C, Lambert N, Javitch JA (2011) CODA-RET reveals functional selectivity as a result of GPCR heteromerization. Nat Chem Biol 7(9):624–630. https://doi.org/10.1038/nchembio.623
Jiang LI, Collins J, Davis R, Lin KM, DeCamp D, Roach T, Hsueh R, Rebres RA et al (2007) Use of a cAMP BRET sensor to characterize a novel regulation of cAMP by the sphingosine 1-phosphate/G13 pathway. J Biol Chem 282(14):10576–10584. https://doi.org/10.1074/jbc.M609695200
Casadó V, Cortés A, Ciruela F, Mallol J, Ferré S, Lluis C, Canela EI, Franco R (2007) Old and new ways to calculate the affinity of agonists and antagonists interacting with G-protein-coupled monomeric and dimeric receptors: the receptor-dimer cooperativity index. Pharmacol Ther 116(3):343–354. https://doi.org/10.1016/j.pharmthera.2007.05.010
Ferré S, Casadó V, Devi LA, Filizola M, Jockers R, Lohse MJ, Milligan G, Pin JP et al (2014) G protein-coupled receptor oligomerization revisited: functional and pharmacological perspectives. Pharmacol Rev 66(2):413–434. https://doi.org/10.1124/pr.113.008052
Casadó V, Ferrada C, Bonaventura J, Gracia E, Mallol J, Canela EI, Lluis C, Cortés A et al (2009) Useful pharmacological parameters for G-protein-coupled receptor homodimers obtained from competition experiments. Agonist-antagonist binding modulation. Biochem Pharmacol 78(12):1456–1463. https://doi.org/10.1016/j.bcp.2009.07.012
Song Y, DiMaio F, Wang RY, Kim D, Miles C, Brunette T, Thompson J, Baker D (2013) High-resolution comparative modeling with RosettaCM. Structure 21(10):1735–1742. https://doi.org/10.1016/j.str.2013.08.005
Bender BJ, Cisneros A 3rd, Duran AM, Finn JA, Fu D, Lokits AD, Mueller BK, Sangha AK et al (2016) Protocols for molecular modeling with Rosetta3 and RosettaScripts. Biochemistry 55(34):4748–4763. https://doi.org/10.1021/acs.biochem.6b00444
Chien EY, Liu W, Zhao Q, Katritch V, Han GW, Hanson MA, Shi L, Newman AH et al (2010) Structure of the human dopamine D3 receptor in complex with a D2/D3 selective antagonist. Science 330(6007):1091–1095. https://doi.org/10.1126/science.1197410
Miller-Gallacher JL, Nehme R, Warne T, Edwards PC, Schertler GF, Leslie AG, Tate CG (2014) The 2.1 A resolution structure of cyanopindolol-bound beta1-adrenoceptor identifies an intramembrane Na+ ion that stabilises the ligand-free receptor. PLoS One 9(3):e92727. https://doi.org/10.1371/journal.pone.0092727
Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P et al (2007) High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science 318(5854):1258–1265. https://doi.org/10.1126/science.1150577
Wang C, Jiang Y, Ma J, Wu H, Wacker D, Katritch V, Han GW, Liu W et al (2013) Structural basis for molecular recognition at serotonin receptors. Science 340(6132):610–614. https://doi.org/10.1126/science.1232807
Wacker D, Wang C, Katritch V, Han GW, Huang XP, Vardy E, McCorvy JD, Jiang Y et al (2013) Structural features for functional selectivity at serotonin receptors. Science 340(6132):615–619. https://doi.org/10.1126/science.1232808
Kothiwale S, Mendenhall JL, Meiler J (2015) BCL::Conf: small molecule conformational sampling using a knowledge based rotamer library. J Cheminform 7:47. https://doi.org/10.1186/s13321-015-0095-1
Meiler J, Baker D (2006) ROSETTALIGAND: protein-small molecule docking with full side-chain flexibility. Proteins 65(3):538–548. https://doi.org/10.1002/prot.21086
Lemmon G, Meiler J (2012) Rosetta Ligand docking with flexible XML protocols. Methods Mol Biol 819:143–155. https://doi.org/10.1007/978-1-61779-465-0_10
Meador-Woodruff JH, Damask SP, Wang J, Haroutunian V, Davis KL Watson SJ (1996) Dopamine receptor mRNA expression in human striatum and neocortex. Neuropsychopharmacology 15:17–29
Pohjanoksa K, Jansson CC, Luomala K, Marjamaki A, Savola JM, Scheinin M (1997) Alpha2-adrenoceptor regulation of adenylyl cyclase in CHO cells: dependence on receptor density, receptor subtype and current activity of adenylyl cyclase. Eur J Pharmacol 335(1):53–63
Kukkonen JP, Renvaktar A, Shariatmadari R, Akerman KE (1998) Ligand- and subtype-selective coupling of human alpha-2 adrenoceptors to Ca++ elevation in Chinese hamster ovary cells. J Pharmacol Exp Ther 287(2):667–671
Schröder R, Schmidt J, Blättermann S, Peters L, Janssen N, Grundmann M, Seemann W, Kaufel D et al (2011) Applying label-free dynamic mass redistribution technology to frame signaling of G protein-coupled receptors noninvasively in living cells. Nat Protoc 6(11):1748–1760. https://doi.org/10.1038/nprot.2011.386
Atwood BK, Lopez J, Wager-Miller J, Mackie K, Straiker A (2011) Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as revealed by microarray analysis. BMC Genomics 12:14. https://doi.org/10.1186/1471-2164-12-14
Fraser CM, Arakawa S, McCombie WR, Venter JC (1989) Cloning, sequence analysis, and permanent expression of a human alpha 2-adrenergic receptor in Chinese hamster ovary cells. Evidence for independent pathways of receptor coupling to adenylate cyclase attenuation and activation. J Biol Chem 264(20):11754–11761
Jones SB, Halenda SP, Bylund DB (1991) Alpha 2-adrenergic receptor stimulation of phospholipase A2 and of adenylate cyclase in transfected Chinese hamster ovary cells is mediated by different mechanisms. Mol Pharmacol 39(2):239–245
Eason MG, Jacinto MT, Liggett SB (1994) Contribution of ligand structure to activation of alpha 2-adrenergic receptor subtype coupling to Gs. Mol Pharmacol 45(4):696–702
Eason MG, Kurose H, Holt BD, Raymond JR, Liggett SB (1992) Simultaneous coupling of alpha 2-adrenergic receptors to two G-proteins with opposing effects. Subtype-selective coupling of alpha 2C10, alpha 2C4, and alpha 2C2 adrenergic receptors to Gi and Gs. J Biol Chem 267(22):15795–11580
Eason MG, Liggett SB (1995) Identification of a Gs coupling domain in the amino terminus of the third intracellular loop of the alpha 2A-adrenergic receptor. Evidence for distinct structural determinants that confer Gs versus Gi coupling. J Biol Chem 270(42):24753–24760
Leaver-Fay A, Tyka M, Lewis SM, Lange OF, Thompson J, Jacak R, Kaufman K, Renfrew PD et al (2011) ROSETTA3: an object-oriented software suite for the simulation and design of macromolecules. Methods Enzymol 487:545–574
Ring AM, Manglik A, Kruse AC, Enos MD, Weis WI, Garcia KC, Kobilka BK (2013) Adrenaline-activated structure of beta2-adrenoceptor stabilized by an engineered nanobody. Nature 502(7472):575–579. https://doi.org/10.1038/nature12572
Feng Z, Hou T, Li Y (2012) Selectivity and activation of dopamine D3R from molecular dynamics. J Mol Model 18(12):5051–5063. https://doi.org/10.1007/s00894-012-1509-x
Owesson-White CA, Roitman MF, Sombers LA, Belle AM, Keithley RB, Peele JL, Carelli RM, Wightman RM (2012) Sources contributing to the average extracellular concentration of dopamine in the nucleus accumbens. J Neurochem 121(2):252–262. https://doi.org/10.1111/j.1471-4159.2012.07677.x
Rice ME, Patel JC, Cragg SJ (2011) Dopamine release in the basal ganglia. Neuroscience 198:112–137. https://doi.org/10.1016/j.neuroscience.2011.08.066
Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, Bolam JP, Ince E et al (1995) Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neurosci 15(7 Pt 2):5222–5237. https://doi.org/10.1016/b978-0-12-381270-4.00019-6
Yung KK, Bolam JP, Smith AD, Hersch SM, Ciliax BJ, Levey AI (1995) Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neuroscience 65(3):709–730
Goldman-Rakic PS, Lidow MS, Smiley JF, Williams MS (1992) The anatomy of dopamine in monkey and human prefrontal cortex. J Neural Transm Suppl 36:163–177
Castelli MP, Spiga S, Perra A, Madeddu C, Mulas G, Ennas MG, Gessa GL (2016) alpha2A adrenergic receptors highly expressed in mesoprefrontal dopamine neurons. Neuroscience 332:130–139. https://doi.org/10.1016/j.neuroscience.2016.06.037
Lee A, Wissekerke AE, Rosin DL, Lynch KR (1998) Localization of alpha2C-adrenergic receptor immunoreactivity in catecholaminergic neurons in the rat central nervous system. Neuroscience 84(4):1085–1096
Brennan AR, Arnsten AF (2008) Neuronal mechanisms underlying attention deficit hyperactivity disorder: the influence of arousal on prefrontal cortical function. Ann N Y Acad Sci 1129:236–245. https://doi.org/10.1196/annals.1417.007
Herve D (2011) Identification of a specific assembly of the g protein golf as a critical and regulated module of dopamine and adenosine-activated cAMP pathways in the striatum. Front Neuroanat 5:48. https://doi.org/10.3389/fnana.2011.00048
Acknowledgements
The authors would like to thank Dr. Céline Galés (INSERM) for generously sharing various Gi-like plasmid constructs for BRET experiments.
Funding
This research was supported by the “Ministerio de Economía y Competitividad” and European Regional Development Funds of the European Union Grant SAF2014-54840-R, the “Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas” Grant CB06/05/0064, the “Fundació La Marató de TV3” Grant 20140610, the fellowship from the Japan Society for the Promotion of Science, and intramural funds of the National Institute on Drug Abuse. Work in the Meiler Laboratory is supported through the NIH (R01 GM080403, R01 GM099842, R01 DK097376) and NSF (CHE 1305874).
Author information
Authors and Affiliations
Contributions
M. S.-S., V. C.-A., H. Y., N.-S. C., E. M., A. C., and B. J. B. performed the experiments and analyzed the data. H. Y., B. J. B., J. M., V. C., and S. F. designed the experiments. M. S.-S., V. C.-A., H. Y., B. J. B., J. M., E. I. C., V. C., and S. F. wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Sánchez-Soto, M., Casadó-Anguera, V., Yano, H. et al. α2A- and α2C-Adrenoceptors as Potential Targets for Dopamine and Dopamine Receptor Ligands. Mol Neurobiol 55, 8438–8454 (2018). https://doi.org/10.1007/s12035-018-1004-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1004-1