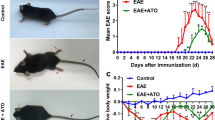
Abstract
Multiple sclerosis (MS) is a T cell autoimmune, inflammatory, and demyelinating disease of the central nervous system (CNS). Currently available therapies have partially effective actions and numerous side reactions. Inosine, an endogenous purine nucleoside, has immunomodulatory, neuroprotective, and analgesic properties. Herein, we evaluated the effect of inosine on the development and progression of experimental autoimmune encephalomyelitis (EAE), an experimental model of MS. Inosine (1 or 10 mg/kg, i.p.) was administrated twice a day for 40 days. Immunological and inflammatory responses were evaluated by behavioral, histological, immunohistochemical, ELISA, RT-PCR, and Western blotting analysis. The administration of inosine exerted neuroprotective effects against EAE by diminishing clinical signs, including thermal and mechanical hyperalgesia, as well as weight loss typical of the disease. These beneficial effects of inosine seem to be associated with the blockade of inflammatory cell entry into the CNS, especially lymphocytes, thus delaying the demyelinating process and astrocytes activation. In particular, up-regulation of IL-17 levels in the secondary lymphoid tissues, a result of EAE, was prevented by inosine treatment in EAE mice. Additionally, inosine consistently prevented A2AR up-regulation in the spinal cord, likely, through an ERK1-independent pathway. Altogether, these results allow us to propose that this endogenous purine might be a putative novel and helpful tool for the prevention of autoimmune and neurodegenerative diseases, such as MS. Thus, inosine could have considerable implications for future therapies of MS, and this study may represent the starting point for further investigation into the role of inosine and adenosinergic receptors in neuroinflammation processes.

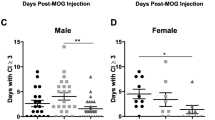
Preventive treatment with inosine inhibits the development and progression of EAE in C57Bl/6 mice. Furthermore, neuroinflammation and demyelinating processes were blocked by inosine treatment. Additionally, inosine consistently inhibited IL-17 levels in peripheral lymphoid tissue, as well as IL-4 levels and A2AR up-regulation in the spinal cord, likely, through an ERK1-independent pathway. EAE: experimental autoimmune encephalomyelitis; MS: multiple sclerosis; A2AR: adenosine A2A receptor; IL-17: interleukin-17; IL-4: interleukin-4







Similar content being viewed by others
References
Nylander A, Hafler DA (2012) Multiple sclerosis. J Clin Invest 122(4):1180–1188. doi:10.1172/JCI58649
Sawcer S, Franklin RJ, Ban M (2014) Multiple sclerosis genetics. Lancet Neurol 13(7):700–709. doi:10.1016/S1474-4422(14)70041-9
Blahova Dusankova J, Kalincik T, Dolezal T, Kobelt G, Havrdova E (2012) Cost of multiple sclerosis in the Czech Republic: the COMS study. Mult Scler 18(5):662–668. doi:10.1177/1352458511424422
Federation MSI (2013) Atlas of MS 2013. http://www.atlasofms.org/. Accessed 23 de maio 2014
Ascherio A, Munger KL, Simon KC (2010) Vitamin D and multiple sclerosis. Lancet Neurol 9(6):599–612. doi:10.1016/S1474-4422(10)70086-7
Goodin DS (2009) The causal cascade to multiple sclerosis: a model for MS pathogenesis. PLoS One 4(2):e4565. doi:10.1371/journal.pone.0004565
McFarland HF, Martin R (2007) Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol 8(9):913–919. doi:10.1038/Ni1507
Compston A, Coles A (2008) Multiple sclerosis. Lancet 372(9648):1502–1517. doi:10.1016/S0140-6736(08)61620-7
Comabella M, Khoury SJ (2012) Immunopathogenesis of multiple sclerosis. Clin Immunol 142(1):2–8. doi:10.1016/j.clim.2011.03.004
Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y (2006) IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol 177(1):566–573
Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, Fugger L (2008) Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol 172(1):146–155. doi:10.2353/ajpath.2008.070690
Moseley TA, Haudenschild DR, Rose L, Reddi AH (2003) Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev 14(2):155–174
Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM (2008) Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med 14(3):337–342. doi:10.1038/nm1715
O’Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH (2008) Pain associated with multiple sclerosis: systematic review and proposed classification. Pain 137(1):96–111. doi:10.1016/j.pain.2007.08.024
Goretti B, Viterbo RG, Portaccio E, Niccolai C, Hakiki B, Piscolla E, Iaffaldano P, Trojano M et al (2014) Anxiety state affects information processing speed in patients with multiple sclerosis. Neurol Sci 35(4):559–563. doi:10.1007/s10072-013-1544-0
Rubin SM (2013) Management of cognition and fatigue. Dis Mon 59(7):269–272. doi:10.1016/j.disamonth.2013.03.014
Gallien P, Gich J, Sanchez-Dalmau BF, Feneberg W (2014) Multidisciplinary management of multiple sclerosis symptoms. Eur Neurol 72(Suppl 1):20–25. doi:10.1159/000367620
Dutra RC, de Souza PR, Bento AF, Marcon R, Bicca MA, Pianowski LF, Calixto JB (2012) Euphol prevents experimental autoimmune encephalomyelitis in mice: evidence for the underlying mechanisms. Biochem Pharmacol 83(4):531–542. doi:10.1016/j.bcp.2011.11.026
Dutra RC, Bento AF, Leite DF, Manjavachi MN, Marcon R, Bicca MA, Pesquero JB, Calixto JB (2013) The role of kinin B1 and B2 receptors in the persistent pain induced by experimental autoimmune encephalomyelitis (EAE) in mice: evidence for the involvement of astrocytes. Neurobiol Dis 54:82–93. doi:10.1016/j.nbd.2013.02.007
Kim CF, Moalem-Taylor G (2011) Interleukin-17 contributes to neuroinflammation and neuropathic pain following peripheral nerve injury in mice. J Pain 12(3):370–383. doi:10.1016/j.jpain.2010.08.003
Dachir S, Shabashov D, Trembovler V, Alexandrovich AG, Benowitz LI, Shohami E (2014) Inosine improves functional recovery after experimental traumatic brain injury. Brain Res 1555:78–88. doi:10.1016/j.brainres.2014.01.044
Kim D, Zai L, Liang P, Schaffling C, Ahlborn D, Benowitz LI (2013) Inosine enhances axon sprouting and motor recovery after spinal cord injury. PLoS One 8(12):e81948. doi:10.1371/journal.pone.0081948
Smith JM, Lunga P, Story D, Harris N, Le Belle J, James MF, Pickard JD, Fawcett JW (2007) Inosine promotes recovery of skilled motor function in a model of focal brain injury. Brain: J Neurol 130(Pt 4):915–925. doi:10.1093/brain/awl393
Zai L, Ferrari C, Subbaiah S, Havton LA, Coppola G, Strittmatter S, Irwin N, Geschwind D et al (2009) Inosine alters gene expression and axonal projections in neurons contralateral to a cortical infarct and improves skilled use of the impaired limb. J Neurosci 29(25):8187–8197. doi:10.1523/JNEUROSCI.0414-09.2009
Hasko G, Sitkovsky MV, Szabo C (2004) Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacol Sci 25(3):152–157. doi:10.1016/j.tips.2004.01.006
da Rocha Lapa F, de Oliveira AP, Accetturi BG, de Oliveira MI, Domingos HV, de Almeida CD, de Lima WT, Santos AR (2013) Anti-inflammatory effects of inosine in allergic lung inflammation in mice: evidence for the participation of adenosine A2A and A 3 receptors. Purinergic signal 9(3):325–336. doi:10.1007/s11302-013-9351-x
da Rocha LF, da Silva MD, de Almeida CD, Santos AR (2012) Anti-inflammatory effects of purine nucleosides, adenosine and inosine, in a mouse model of pleurisy: evidence for the role of adenosine A2 receptors. Purinergic Signal 8(4):693–704. doi:10.1007/s11302-012-9299-2
Nascimento FP, Figueredo SM, Marcon R, Martins DF, Macedo SJ Jr, Lima DA, Almeida RC, Ostroski RM et al (2010) Inosine reduces pain-related behavior in mice: involvement of adenosine A1 and A2A receptor subtypes and protein kinase C pathways. J Pharmacol Exp Ther 334(2):590–598. doi:10.1124/jpet.110.166058
Nascimento FP, Macedo-Junior SJ, Pamplona FA, Luiz-Cerutti M, Cordova MM, Constantino L, Tasca CI, Dutra RC et al (2014) Adenosine A1 receptor-dependent antinociception induced by inosine in mice: pharmacological. Genetic Biochem Aspects Mol Neurobiol. doi:10.1007/s12035-014-8815-5
Kaster MP, Budni J, Gazal M, Cunha MP, Santos AR, Rodrigues AL (2013) The antidepressant-like effect of inosine in the FST is associated with both adenosine A1 and A 2A receptors. Purinergic Signal 9(3):481–486. doi:10.1007/s11302-013-9361-8
Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J (2001) International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53(4):527–552
Buckley S, Barsky L, Weinberg K, Warburton D (2005) In vivo inosine protects alveolar epithelial type 2 cells against hyperoxia-induced DNA damage through MAP kinase signaling. Am J Physiol Lung Cell Mol Physiol 288(3):L569–L575. doi:10.1152/ajplung.00278.2004
Tomaselli B, Nedden SZ, Podhraski V, Baier-Bitterlich G (2008) p42/44 MAPK is an essential effector for purine nucleoside-mediated neuroprotection of hypoxic PC12 cells and primary cerebellar granule neurons. Mol Cell Neurosci 38(4):559–568. doi:10.1016/j.mcn.2008.05.004
Zhang Y, Blattman JN, Kennedy NJ, Duong J, Nguyen T, Wang Y, Davis RJ, Greenberg PD et al (2004) Regulation of innate and adaptive immune responses by MAP kinase phosphatase 5. Nature 430(7001):793–797. doi:10.1038/nature02764
Agrawal A, Dillon S, Denning TL, Pulendran B (2006) ERK1−/− mice exhibit Th1 cell polarization and increased susceptibility to experimental autoimmune encephalomyelitis. J Immunol 176(10):5788–5796
Markowitz CE, Spitsin S, Zimmerman V, Jacobs D, Udupa JK, Hooper DC, Koprowski H (2009) The treatment of multiple sclerosis with inosine. J Altern Complement Med 15(6):619–625. doi:10.1089/acm.2008.0513
Toncev G (2006) Therapeutic value of serum uric acid levels increasing in the treatment of multiple sclerosis. Vojnosanitetski Pregled Military-Med Pharmaceut Rev 63(10):879–882
Mills JH, Kim DG, Krenz A, Chen JF, Bynoe MS (2012) A2A adenosine receptor signaling in lymphocytes and the central nervous system regulates inflammation during experimental autoimmune encephalomyelitis. J Immunol 188(11):5713–5722. doi:10.4049/jimmunol.1200545
Stromnes IM, Goverman JM (2006) Active induction of experimental allergic encephalomyelitis. Nat Protoc 1(4):1810–1819. doi:10.1038/nprot.2006.285
Lu J, Kurejova M, Wirotanseng LN, Linker RA, Kuner R, Tappe-Theodor A (2012) Pain in experimental autoimmune encephalitis: a comparative study between different mouse models. J Neuroinflammation 9:233. doi:10.1186/1742-2094-9-233
Walczak JS, Beaulieu P (2006) Comparison of three models of neuropathic pain in mice using a new method to assess cold allodynia: the double plate technique. Neurosci Lett 399(3):240–244. doi:10.1016/j.neulet.2006.01.058
Lappas CM, Rieger JM, Linden J (2005) A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol 174(2):1073–1080
Su KY, Yu CY, Chen YW, Huang YT, Chen CT, Wu HF, Chen YL (2014) Rutin, a flavonoid and principal component of saussurea involucrata, attenuates physical fatigue in a forced swimming mouse model. Int J Med Sci 11(5):528–537. doi:10.7150/ijms.8220
Gajofatto A, Bacchetti P, Grimes B, High A, Waubant E (2009) Switching first-line disease-modifying therapy after failure: impact on the course of relapsing-remitting multiple sclerosis. Mult Scler 15(1):50–58. doi:10.1177/1352458508096687
Ouyang S, Hsuchou H, Kastin AJ, Mishra PK, Wang Y, Pan W (2014) Leukocyte infiltration into spinal cord of EAE mice is attenuated by removal of endothelial leptin signaling. Brain Behav Immun 40:61–73. doi:10.1016/j.bbi.2014.02.003
Dutra RC, Leite DF, Bento AF, Manjavachi MN, Patricio ES, Figueiredo CP, Pesquero JB, Calixto JB (2011) The role of kinin receptors in preventing neuroinflammation and its clinical severity during experimental autoimmune encephalomyelitis in mice. PLoS One 6(11):e27875. doi:10.1371/journal.pone.0027875
Brambilla R, Morton PD, Ashbaugh JJ, Karmally S, Lambertsen KL, Bethea JR (2014) Astrocytes play a key role in EAE pathophysiology by orchestrating in the CNS the inflammatory response of resident and peripheral immune cells and by suppressing remyelination. Glia 62(3):452–467. doi:10.1002/glia.22616
Hedegaard CJ, Krakauer M, Bendtzen K, Lund H, Sellebjerg F, Nielsen CH (2008) T helper cell type 1 (Th1), Th2 and Th17 responses to myelin basic protein and disease activity in multiple sclerosis. Immunology 125(2):161–169. doi:10.1111/j.1365-2567.2008.02837.x
Tsutsui S, Schnermann J, Noorbakhsh F, Henry S, Yong VW, Winston BW, Warren K, Power C (2004) A1 adenosine receptor upregulation and activation attenuates neuroinflammation and demyelination in a model of multiple sclerosis. J Neurosci 24(6):1521–1529. doi:10.1523/JNEUROSCI.4271-03.2004
Vincenzi F, Corciulo C, Targa M, Merighi S, Gessi S, Casetta I, Gentile M, Granieri E et al (2013) Multiple sclerosis lymphocytes upregulate A2A adenosine receptors that are antiinflammatory when stimulated. Eur J Immunol 43(8):2206–2216. doi:10.1002/eji.201343314
Shin T, Ahn M, Jung K, Heo S, Kim D, Jee Y, Lim YK, Yeo EJ (2003) Activation of mitogen-activated protein kinases in experimental autoimmune encephalomyelitis. J Neuroimmunol 140(1–2):118–125
Hauser SL GD (2008) Multiple sclerosis and other demyelinating diseases. In: Harrison’s Principles of Internal Medicine, vol II. McGraw-Hill Medical, New York, pp 2611–2621
Acharjee S, Nayani N, Tsutsui M, Hill MN, Ousman SS, Pittman QJ (2013) Altered cognitive-emotional behavior in early experimental autoimmune encephalitis—cytokine and hormonal correlates. Brain Behav Immun 33:164–172. doi:10.1016/j.bbi.2013.07.003
Peruga I, Hartwig S, Thone J, Hovemann B, Gold R, Juckel G, Linker RA (2011) Inflammation modulates anxiety in an animal model of multiple sclerosis. Behav Brain Res 220(1):20–29. doi:10.1016/j.bbr.2011.01.018
Bermel RA, You X, Foulds P, Hyde R, Simon JH, Fisher E, Rudick RA (2013) Predictors of long-term outcome in multiple sclerosis patients treated with interferon beta. Ann Neurol 73(1):95–103. doi:10.1002/ana.23758
Rissanen E, Virta JR, Paavilainen T, Tuisku J, Helin S, Luoto P, Parkkola R, Rinne JO et al (2013) Adenosine A2A receptors in secondary progressive multiple sclerosis: a [(11)C]TMSX brain PET study. J Cereb Blood Flow Metab 33(9):1394–1401. doi:10.1038/jcbfm.2013.85
Tsutsui S, Vergote D, Shariat N, Warren K, Ferguson SS, Power C (2008) Glucocorticoids regulate innate immunity in a model of multiple sclerosis: reciprocal interactions between the A1 adenosine receptor and beta-arrestin-1 in monocytoid cells. FASEB J 22(3):786–796. doi:10.1096/fj.07-9002com
Kean RB, Spitsin SV, Mikheeva T, Scott GS, Hooper DC (2000) The peroxynitrite scavenger uric acid prevents inflammatory cell invasion into the central nervous system in experimental allergic encephalomyelitis through maintenance of blood-central nervous system barrier integrity. J Immunol 165(11):6511–6518
Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N et al (2007) Human TH17 lymphocytes promote blood–brain barrier disruption and central nervous system inflammation. Nat Med 13(10):1173–1175. doi:10.1038/nm1651
Zhang GX, Gran B, Yu S, Li J, Siglienti I, Chen X, Kamoun M, Rostami A (2003) Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-beta 2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. J Immunol 170(4):2153–2160
Pitt D, Werner P, Raine CS (2000) Glutamate excitotoxicity in a model of multiple sclerosis. Nat Med 6(1):67–70. doi:10.1038/71555
Carlson T, Kroenke M, Rao P, Lane TE, Segal B (2008) The Th17-ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J Exp Med 205(4):811–823. doi:10.1084/jem.20072404
Brereton CF, Sutton CE, Lalor SJ, Lavelle EC, Mills KH (2009) Inhibition of ERK MAPK suppresses IL-23- and IL-1-driven IL-17 production and attenuates autoimmune disease. J Immunol 183(3):1715–1723. doi:10.4049/jimmunol.0803851
Osterberg A, Boivie J (2010) Central pain in multiple sclerosis—sensory abnormalities. Eur J Pain 14(1):104–110. doi:10.1016/j.ejpain.2009.03.003
Day YJ, Liou JT, Lee CM, Lin YC, Mao CC, Chou AH, Liao CC, Lee HC (2014) Lack of interleukin-17 leads to a modulated micro-environment and amelioration of mechanical hypersensitivity after peripheral nerve injury in mice. Pain 155(7):1293–1302. doi:10.1016/j.pain.2014.04.004
Muto J, Lee H, Lee H, Uwaya A, Park J, Nakajima S, Nagata K, Ohno M et al (2014) Oral administration of inosine produces antidepressant-like effects in mice. Sci Rep 4:4199. doi:10.1038/srep04199
Hughes RN, Hancock NJ, Henwood GA, Rapley SA (2014) Evidence for anxiolytic effects of acute caffeine on anxiety-related behavior in male and female rats tested with and without bright light. Behav Brain Res 271:7–15. doi:10.1016/j.bbr.2014.05.038
Kulkarni SK, Singh K, Bishnoi M (2007) Involvement of adenosinergic receptors in anxiety related behaviours. Indian J Exp Biol 45(5):439–443
Kroencke DC, Lynch SG, Denney DR (2000) Fatigue in multiple sclerosis: relationship to depression, disability, and disease pattern. Mult Scler 6(2):131–136
Flachenecker P, Bihler I, Weber F, Gottschalk M, Toyka KV, Rieckmann P (2004) Cytokine mRNA expression in patients with multiple sclerosis and fatigue. Mult Scler 10(2):165–169
Acknowledgments
We thank Gabriela Segat, Thaís Barbosa Alberti, Rodrigo Marcon and Allisson Freire Bento for technical assistance. Grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Apoio a Pesquisa do Estado de Santa Catarina (FAPESC), and Programa de Pós-Graduação em Neurociências (PGN), all from Brazil, supported this work. S.C.J., I.S.C and V.L. are PhD students in neuroscience receiving grants from CAPES and FAPESC. M.P.C. holds a postdoctoral fellowship from CAPES.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
There are no conflicts of interest.
Additional information
Highlights
• Inosine displays pronounced anti-inflammatory and antinociceptive properties.
• Inosine inhibits demyelinating process and astroglial activation.
• Inosine modulates adenosinergic receptors, especially A2AR.
Rights and permissions
About this article
Cite this article
Junqueira, S.C., dos Santos Coelho, I., Lieberknecht, V. et al. Inosine, an Endogenous Purine Nucleoside, Suppresses Immune Responses and Protects Mice from Experimental Autoimmune Encephalomyelitis: a Role for A2A Adenosine Receptor. Mol Neurobiol 54, 3271–3285 (2017). https://doi.org/10.1007/s12035-016-9893-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9893-3