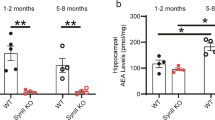
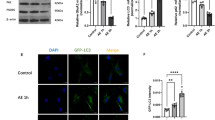
Abstract
In the brain, astrocytes signal to neighboring cells via regulated exocytotic release of gliosignaling molecules, such as brain-derived neurotrophic factor (BDNF). Recent studies uncovered a role of ketamine, an anesthetic and antidepressant, in the regulation of BDNF expression and in the disruption of astrocytic Ca2+ signaling, but it is unclear whether it affects astroglial BDNF release. We investigated whether ketamine affects ATP-evoked Ca2+ signaling and exocytotic release of BDNF at the single-vesicle level in cultured rat astrocytes. Cells were transfected with a plasmid encoding preproBDNF tagged with the pH-sensitive fluorescent protein superecliptic pHluorin, (BDNF-pHse) to load vesicles and measure the release of BDNF-pHse when the exocytotic fusion pore opens and alkalinizes the luminal pH. In addition, cell-attached membrane capacitance changes were recorded to monitor unitary vesicle interaction with the plasma membrane. Intracellular Ca2+ activity was monitored with Fluo-4 and confocal microscopy, which was also used to immunocytochemically characterize BDNF-pHse-laden vesicles. As revealed by double-fluorescent micrographs, BDNF-pHse localized to vesicles positive for the soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor (SNARE) proteins, vesicle-associated membrane protein 2 (VAMP2), VAMP3, and synaptotagmin IV. Ketamine treatment decreased the number of ATP-evoked BDNF-pHse fusion/secretion events (P < 0.05), the frequency of ATP-evoked transient (P < 0.001) and full-fusion exocytotic (P < 0.05) events, along with a reduction in the ATP-evoked increase in intracellular Ca2+ activity in astrocytes by ~70 % (P < 0.001). The results show that ketamine treatment suppresses ATP-triggered vesicle fusion and BDNF secretion by increasing the probability of a narrow fusion pore open state and/or by reducing astrocytic Ca2+ excitability.






Similar content being viewed by others
References
Lessmann V, Brigadski T (2009) Mechanisms, locations, and kinetics of synaptic BDNF secretion: an update. Neurosci Res 65:11–22. doi:10.1016/j.neures.2009.06.004
Allaman I, Fiumelli H, Magistretti PJ, Martin JL (2011) Fluoxetine regulates the expression of neurotrophic/growth factors and glucose metabolism in astrocytes. Psychopharmacol (Berl) 216:75–84. doi:10.1007/s00213-011-2190-y
Moretto G, Xu RY, Walker DG, Kim SU (1994) Co-expression of mRNA for neurotrophic factors in human neurons and glial cells in culture. J Neuropathol Exp Neurol 53:78–85
Toyomoto M, Inoue S, Ohta K, Kuno S, Ohta M, Hayashi K, Ikeda K (2005) Production of NGF, BDNF and GDNF in mouse astrocyte cultures is strongly enhanced by a cerebral vasodilator, ifenprodil. Neurosci Lett 379:185–189. doi:10.1016/j.neulet.2004.12.063
Wu H, Friedman WJ, Dreyfus CF (2004) Differential regulation of neurotrophin expression in basal forebrain astrocytes by neuronal signals. J Neurosci Res 76:76–85. doi:10.1002/jnr.20060
Zafra F, Lindholm D, Castrén E, Hartikka J, Thoenen H (1992) Regulation of brain-derived neurotrophic factor and nerve growth factor mRNA in primary cultures of hippocampal neurons and astrocytes. J Neurosci 12:4793–4799
Jean YY, Lercher LD, Dreyfus CF (2008) Glutamate elicits release of BDNF from basal forebrain astrocytes in a process dependent on metabotropic receptors and the PLC pathway. Neuron Glia Biol 4:35–42. doi:10.1017/S1740925X09000052
Rudge JS, Pasnikowski EM, Holst P, Lindsay RM (1995) Changes in neurotrophic factor expression and receptor activation following exposure of hippocampal neuron/astrocyte cocultures to kainic acid. J Neurosci 15:6856–6867
Inoue S, Susukida M, Ikeda K, Murase K, Hayashi K (1997) Dopaminergic transmitter up-regulation of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) synthesis in mouse astrocytes in culture. Biochem Biophys Res Commun 238:468–472. doi:10.1006/bbrc.1997.7324
Fulmer CG, VonDran MW, Stillman AA, Huang Y, Hempstead BL, Dreyfus CF (2014) Astrocyte-derived BDNF supports myelin protein synthesis after cuprizone-induced demyelination. J Neurosci 34:8186–8196. doi:10.1523/JNEUROSCI.4267-13.2014
Giralt A, Friedman HC, Caneda-Ferrón B, Urbán N, Moreno E, Rubio N, Blanco J, Peterson A et al (2010) BDNF regulation under GFAP promoter provides engineered astrocytes as a new approach for long-term protection in Huntington's disease. Gene Ther 17:1294–1308. doi:10.1038/gt.2010.71
Quesseveur G, David DJ, Gaillard MC, Pla P, Wu MV, Nguyen HT, Nicolas V, Auregan G et al (2013) BDNF overexpression in mouse hippocampal astrocytes promotes local neurogenesis and elicits anxiolytic-like activities. Transl Psychiatry 3, e253. doi:10.1038/tp.2013.30
Prickaerts J, De Vry J, Boere J, Kenis G, Quinton MS, Engel S, Melnick L, Schreiber R (2012) Differential BDNF responses of triple versus dual reuptake inhibition in neuronal and astrocytoma cells as well as in rat hippocampus and prefrontal cortex. J Mol Neurosci 48:167–175. doi:10.1007/s12031-012-9802-9
Ubhi K, Inglis C, Mante M, Patrick C, Adame A, Spencer B, Rockenstein E, May V et al (2012) Fluoxetine ameliorates behavioral and neuropathological deficits in a transgenic model mouse of alpha-synucleinopathy. Exp Neurol 234:405–416. doi:10.1016/j.expneurol.2012.01.008
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475:91–95. doi:10.1038/nature10130
Serafini G, Howland RH, Rovedi F, Girardi P, Amore M (2014) The role of ketamine in treatment-resistant depression: a systematic review. Curr Neuropharmacol 12:444–461. doi:10.2174/1570159X12666140619204251
Akinfiresoye L, Tizabi Y (2013) Antidepressant effects of AMPA and ketamine combination: role of hippocampal BDNF, synapsin, and mTOR. Psychopharmacol (Berl) 230:291–298. doi:10.1007/s00213-013-3153-2
Fraga DB, Réus GZ, Abelaira HM, De Luca RD, Canever L, Pfaffenseller B, Colpo GD, Kapczinski F et al (2013) Ketamine alters behavior and decreases BDNF levels in the rat brain as a function of time after drug administration. Rev Bras Psiquiatr 35:262–266. doi:10.1590/1516-4446-2012-0858
Nosyreva E, Szabla K, Autry AE, Ryazanov AG, Monteggia LM, Kavalali ET (2013) Acute suppression of spontaneous neurotransmission drives synaptic potentiation. J Neurosci 33:6990–7002. doi:10.1523/JNEUROSCI.4998-12.2013
Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK (2012) Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry 71:996–1005. doi:10.1016/j.biopsych.2011.09.030
Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B et al (2003) The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112:257–269
Müller HK, Wegener G, Liebenberg N, Zarate CA, Popoli M, Elfving B (2013) Ketamine regulates the presynaptic release machinery in the hippocampus. J Psychiatr Res 47:892–899. doi:10.1016/j.jpsychires.2013.03.008
Wesseling H, Rahmoune H, Tricklebank M, Guest PC, Bahn S (2015) A targeted multiplexed proteomic investigation identifies ketamine-induced changes in immune markers in rat serum and expression changes in protein kinases/phosphatases in rat brain. J Proteome Res 14:411–421. doi:10.1021/pr5009493
Denovan-Wright EM, Newton RA, Armstrong JN, Babity JM, Robertson HA (1998) Acute administration of cocaine, but not amphetamine, increases the level of synaptotagmin IV mRNA in the dorsal striatum of rat. Brain Res Mol Brain Res 55:350–354
Peng W, Premkumar A, Mossner R, Fukuda M, Lesch KP, Simantov R (2002) Synaptotagmin I and IV are differentially regulated in the brain by the recreational drug 3,4-methylenedioxymethamphetamine (MDMA). Brain Res Mol Brain Res 108:94–101
Dean C, Liu H, Dunning FM, Chang PY, Jackson MB, Chapman ER (2009) Synaptotagmin-IV modulates synaptic function and long-term potentiation by regulating BDNF release. Nat Neurosci 12:767–776. doi:10.1038/nn.2315
Stenovec M, Trkov S, Kreft M, Zorec R (2014) Alterations of calcium homoeostasis in cultured rat astrocytes evoked by bioactive sphingolipids. Acta Physiol (Oxf) 212:49–61. doi:10.1111/apha.12314
Yang H, Liang G, Hawkins BJ, Madesh M, Pierwola A, Wei H (2008) Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology 109:243–250. doi:10.1097/ALN.0b013e31817f5c47
Thrane AS, Rangroo Thrane V, Zeppenfeld D, Lou N, Xu Q, Nagelhus EA, Nedergaard M (2012) General anesthesia selectively disrupts astrocyte calcium signaling in the awake mouse cortex. Proc Natl Acad Sci U S A 109:18974–18979. doi:10.1073/pnas.1209448109
Bergami M, Santi S, Formaggio E, Cagnoli C, Verderio C, Blum R, Berninger B, Matteoli M et al (2008) Uptake and recycling of pro-BDNF for transmitter-induced secretion by cortical astrocytes. J Cell Biol 183:213–221. doi:10.1083/jcb.200806137
Miesenböck G, De Angelis DA, Rothman JE (1998) Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394:192–195. doi:10.1038/28190
Schwartz JP, Wilson DJ (1992) Preparation and characterization of type 1 astrocytes cultured from adult rat cortex, cerebellum, and striatum. Glia 5:75–80. doi:10.1002/glia.440050111
Jorgacevski J, Stenovec M, Kreft M, Bajic A, Rituper B, Vardjan N, Stojilkovic S, Zorec R (2008) Hypotonicity and peptide discharge from a single vesicle. Am J physiol Cell physiol 295:C624–631. doi:10.1152/ajpcell.00303.2008
Zhang Q, Fukuda M, Van Bockstaele E, Pascual O, Haydon PG (2004) Synaptotagmin IV regulates glial glutamate release. Proc Natl Acad Sci U S A 101:9441–9446. doi:10.1073/pnas.0401960101
Hepp R, Perraut M, Chasserot-Golaz S, Galli T, Aunis D, Langley K, Grant NJ (1999) Cultured glial cells express the SNAP-25 analogue SNAP-23. Glia 27:181–187
Parpura V, Fang Y, Basarsky T, Jahn R, Haydon PG (1995) Expression of synaptobrevin II, cellubrevin and syntaxin but not SNAP-25 in cultured astrocytes. FEBS Lett 377:489–492. doi:10.1016/0014-5793(95)01401-2
Gentet LJ, Stuart GJ, Clements JD (2000) Direct measurement of specific membrane capacitance in neurons. Biophys J 79:314–320. doi:10.1016/S0006-3495(00)76293-X
Roos A, Boron WF (1981) Intracellular pH. Physiol Rev 61(2):296–434
Vardjan N, Stenovec M, Jorgacevski J, Kreft M, Zorec R (2007) Subnanometer fusion pores in spontaneous exocytosis of peptidergic vesicles. J Neurosci 27:4737–4746. doi:10.1523/JNEUROSCI.0351-07.2007
Mowla SJ, Farhadi HF, Pareek S, Atwal JK, Morris SJ, Seidah NG, Murphy RA (2001) Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J Biol Chem 276:12660–12666. doi:10.1074/jbc.M008104200
Pangrsic T, Potokar M, Haydon PG, Zorec R, Kreft M (2006) Astrocyte swelling leads to membrane unfolding, not membrane insertion. J Neurochem 99:514–523. doi:10.1111/j.1471-4159.2006.04042.x
Stenovec M, Trkov S, Kreft M, Zorec R (2014) Alterations of calcium homeostasis in cultured rat astrocytes evoked by bioactive sphingolipids. Acta Physiol (Oxf). doi:10.1111/apha.12314
Neher E, Marty A (1982) Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A 79:6712–6716
Rituper B, Guček A, Jorgačevski J, Flašker A, Kreft M, Zorec R (2013) High-resolution membrane capacitance measurements for the study of exocytosis and endocytosis. Nat Protoc 8:1169–1183. doi:10.1038/nprot.2013.069
Czeh B, Di Benedetto B (2013) Antidepressants act directly on astrocytes: evidences and functional consequences. Eur Neuropsychopharmacol 23:171–185. doi:10.1016/j.euroneuro.2012.04.017
Gucek A, Vardjan N, Zorec R (2012) Exocytosis in astrocytes: transmitter release and membrane signal regulation. Neurochem Res 37:2351–2363. doi:10.1007/s11064-012-0773-6
Krzan M, Stenovec M, Kreft M, Pangrsic T, Grilc S, Haydon PG, Zorec R (2003) Calcium-dependent exocytosis of atrial natriuretic peptide from astrocytes. J Neurosci 23:1580–1583
Malarkey EB, Parpura V (2011) Temporal characteristics of vesicular fusion in astrocytes: examination of synaptobrevin 2-laden vesicles at single vesicle resolution. J Physiol 589:4271–4300. doi:10.1113/jphysiol.2011.210435
Ramamoorthy P, Whim MD (2008) Trafficking and fusion of neuropeptide Y-containing dense-core granules in astrocytes. J Neurosci 28:13815–13827. doi:10.1523/JNEUROSCI.5361-07.2008
Zorec R, Araque A, Carmignoto G, Haydon PG, Verkhratsky A, Parpura V (2012) Astroglial excitability and gliotransmission: an appraisal of Ca2+ as a signalling route. ASN neuro 4 (2). 10.1042/AN20110061
Haubensak W, Narz F, Heumann R, Lessmann V (1998) BDNF-GFP containing secretory granules are localized in the vicinity of synaptic junctions of cultured cortical neurons. J Cell Sci 111:1483–1493
Wang CT, Grishanin R, Earles CA, Chang PY, Martin TF, Chapman ER, Jackson MB (2001) Synaptotagmin modulation of fusion pore kinetics in regulated exocytosis of dense-core vesicles. Science 294:1111–1115. doi:10.1126/science.1064002
Wang CT, Lu JC, Bai J, Chang PY, Martin TF, Chapman ER, Jackson MB (2003) Different domains of synaptotagmin control the choice between kiss-and-run and full fusion. Nature 424:943–947. doi:10.1038/nature01857
Pawlu C, DiAntonio A, Heckmann M (2004) Postfusional control of quantal current shape. Neuron 42:607–618
Zhang Z, Jackson MB (2010) Synaptotagmin IV modulation of vesicle size and fusion pores in PC12 cells. Biophys J 98:968–978. doi:10.1016/j.bpj.2009.11.024
Shinoda Y, Sadakata T, Nakao K, Katoh-Semba R, Kinameri E, Furuya A, Yanagawa Y, Hirase H et al (2011) Calcium-dependent activator protein for secretion 2 (CAPS2) promotes BDNF secretion and is critical for the development of GABAergic interneuron network. Proc Natl Acad Sci U S A 108:373–378. doi:10.1073/pnas.1012220108
Stenovec M, Kreft M, Poberaj I, Betz WJ, Zorec R (2004) Slow spontaneous secretion from single large dense-core vesicles monitored in neuroendocrine cells. FASEB J 18:1270–1272. doi:10.1096/fj.03-1397fje
Kojima M, Takei N, Numakawa T, Ishikawa Y, Suzuki S, Matsumoto T, Katoh-Semba R, Nawa H et al (2001) Biological characterization and optical imaging of brain-derived neurotrophic factor-green fluorescent protein suggest an activity-dependent local release of brain-derived neurotrophic factor in neurites of cultured hippocampal neurons. J Neurosci Res 64:1–10
Trkov S, Stenovec M, Kreft M, Potokar M, Parpura V, Davletov B, Zorec R (2012) Fingolimod—a sphingosine-like molecule inhibits vesicle mobility and secretion in astrocytes. Glia 60:1406–1416. doi:10.1002/glia.22361
Perrais D, Kleppe IC, Taraska JW, Almers W (2004) Recapture after exocytosis causes differential retention of protein in granules of bovine chromaffin cells. J Physiol 560:413–428. doi:10.1113/jphysiol.2004.064410
Taraska JW, Perrais D, Ohara-Imaizumi M, Nagamatsu S, Almers W (2003) Secretory granules are recaptured largely intact after stimulated exocytosis in cultured endocrine cells. Proc Natl Acad Sci U S A 100:2070–2075. doi:10.1073/pnas.0337526100
Flašker A, Jorgačevski J, Calejo AI, Kreft M, Zorec R (2013) Vesicle size determines unitary exocytic properties and their sensitivity to sphingosine. Mol Cell Endocrinol 376:136–147. doi:10.1016/j.mce.2013.06.012
Rupnik M, Kreft M, Sikdar SK, Grilc S, Romih R, Zupancic G, Martin TF, Zorec R (2000) Rapid regulated dense-core vesicle exocytosis requires the CAPS protein. Proc Natl Acad Sci U S A 97:5627–5632. doi:10.1073/pnas.090359097
Han W, Ng YK, Axelrod D, Levitan ES (1999) Neuropeptide release by efficient recruitment of diffusing cytoplasmic secretory vesicles. Proc Natl Acad Sci U S A 96:14577–14582
Brigadski T, Hartmann M, Lessmann V (2005) Differential vesicular targeting and time course of synaptic secretion of the mammalian neurotrophins. J Neurosci 25:7601–7614. doi:10.1523/JNEUROSCI.1776-05.2005
Stenovec M, Solmajer T, Perdih A, Vardjan N, Kreft M, Zorec R (2007) Distinct labelling of fusion events in rat lactotrophs by FM 1–43 and FM 4–64 is associated with conformational differences. Acta Physiol (Oxf) 191:35–42. doi:10.1111/j.1748-1716.2007.01716.x
Bandmann V, Kreft M, Homann U (2011) Modes of exocytotic and endocytotic events in tobacco BY-2 protoplasts. Mol Plant 4:241–251. doi:10.1093/mp/ssq072
Thiel G, Kreft M, Zorec R (2009) Rhythmic kinetics of single fusion and fission in a plant cell protoplast. Ann N Y Acad Sci 1152:1–6. doi:10.1111/j.1749-6632.2008.03996.x
Staal RG, Mosharov EV, Sulzer D (2004) Dopamine neurons release transmitter via a flickering fusion pore. Nat Neurosci 7:341–346. doi:10.1038/nn1205
Zhou Z, Misler S, Chow RH (1996) Rapid fluctuations in transmitter release from single vesicles in bovine adrenal chromaffin cells. Biophys J 70:1543–1552. doi:10.1016/S0006-3495(96)79718-7
Henkel AW, Meiri H, Horstmann H, Lindau M, Almers W (2000) Rhythmic opening and closing of vesicles during constitutive exo- and endocytosis in chromaffin cells. EMBO J 19:84–93. doi:10.1093/emboj/19.1.84
Henkel AW, Kang G, Kornhuber J (2001) A common molecular machinery for exocytosis and the 'kiss-and-run' mechanism in chromaffin cells is controlled by phosphorylation. J Cell Sci 114:4613–4620
Leng G, Ludwig M (2008) Neurotransmitters and peptides: whispered secrets and public announcements. J Physiol 586:5625–5632. doi:10.1113/jphysiol.2008.159103
Barg S, Olofsson CS, Schriever-Abeln J, Wendt A, Gebre-Medhin S, Renstrom E, Rorsman P (2002) Delay between fusion pore opening and peptide release from large dense-core vesicles in neuroendocrine cells. Neuron 33:287–299
Harata NC, Choi S, Pyle JL, Aravanis AM, Tsien RW (2006) Frequency-dependent kinetics and prevalence of kiss-and-run and reuse at hippocampal synapses studied with novel quenching methods. Neuron 49:243–256. doi:10.1016/j.neuron.2005.12.018
Kolarow R, Brigadski T, Lessmann V (2007) Postsynaptic secretion of BDNF and NT-3 from hippocampal neurons depends on calcium calmodulin kinase II signaling and proceeds via delayed fusion pore opening. J Neurosci 27:10350–10364. doi:10.1523/JNEUROSCI.0692-07.2007
Park H, Poo MM (2013) Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14:7–23. doi:10.1038/nrn3379
Sudhof TC (2004) The synaptic vesicle cycle. Annu Rev Neurosci 27:509–547. doi:10.1146/annurev.neuro.26.041002.131412
Soldati T, Schliwa M (2006) Powering membrane traffic in endocytosis and recycling. Nat Rev Mol Cell Biol 7:897–908. doi:10.1038/nrm2060
Chang HC, Chen TL, Chen RM (2009) Cytoskeleton interruption in human hepatoma HepG2 cells induced by ketamine occurs possibly through suppression of calcium mobilization and mitochondrial function. Drug Metab Dispos: Biol Fate Chem 37:24–31. doi:10.1124/dmd.108.023325
Koizumi S, Fujishita K, Tsuda M, Shigemoto-Mogami Y, Inoue K (2003) Dynamic inhibition of excitatory synaptic transmission by astrocyte-derived ATP in hippocampal cultures. Proc Natl Acad Sci U S A 100:11023–11028. doi:10.1073/pnas.1834448100
Cotrina ML, Lin JH, Nedergaard M (1998) Cytoskeletal assembly and ATP release regulate astrocytic calcium signaling. J Neurosci 18:8794–8804
Abdelhamid RE, Kovács KJ, Nunez MG, Larson AA (2014) Depressive behavior in the forced swim test can be induced by TRPV1 receptor activity and is dependent on NMDA receptors. Pharmacol Res 79:21–27. doi:10.1016/j.phrs.2013.10.006
Bahnasi YM, Wright HM, Milligan CJ, Dedman AM, Zeng F, Hopkins PM, Bateson AN, Beech DJ (2008) Modulation of TRPC5 cation channels by halothane, chloroform and propofol. Br J Pharmacol 153:1505–1512. doi:10.1038/sj.bjp.0707689
Malarkey EB, Ni Y, Parpura V (2008) Ca2+ entry through TRPC1 channels contributes to intracellular Ca2+ dynamics and consequent glutamate release from rat astrocytes. Glia 56:821–835. doi:10.1002/glia.20656
Verkhratsky A, Rodriguez JJ, Parpura V (2012) Calcium signalling in astroglia. Mol Cell Endocrinol 353:45–56. doi:10.1016/j.mce.2011.08.039
Li B, Dong L, Fu H, Wang B, Hertz L, Peng L (2011) Effects of chronic treatment with fluoxetine on receptor-stimulated increase of [Ca2+]i in astrocytes mimic those of acute inhibition of TRPC1 channel activity. Cell Calcium 50:42–53. doi:10.1016/j.ceca.2011.05.001
Tóth A, Boczán J, Kedei N, Lizanecz E, Bagi Z, Papp Z, Edes I, Csiba L et al (2005) Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res Mol Brain Res 135:162–168. doi:10.1016/j.molbrainres.2004.12.003
Mannari T, Morita S, Furube E, Tominaga M, Miyata S (2013) Astrocytic TRPV1 ion channels detect blood-borne signals in the sensory circumventricular organs of adult mouse brains. Glia 61:957–971. doi:10.1002/glia.22488
Chang Y, Chen TL, Sheu JR, Chen RM (2005) Suppressive effects of ketamine on macrophage functions. Toxicol Appl Pharmacol 204:27–35. doi:10.1016/j.taap.2004.08.011
Acknowledgments
This work was supported by the Slovenian Research Agency grant nos. P3 310, J3 3236, J3 4051, J3-4146, J3 6790. V.P.’s work is supported by the National Institutes of Health (HD078678). We thank Dr. Masami Kojima and Dr. James E. Rothman for kindly providing the preproBDNF-EGFP and pCMV-SpHse plasmids, respectively. All DNA plasmids were sequenced at The Heflin Center Genomics Core at UAB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Exocytotic release of brain-derived neurotrophic factor (BDNF). BDNF-pHse, a fusion protein of BDNF and superecliptic phluorin (pHse), allowed us to monitor its release. ATP stimulation induces fusion of vesicles at the plasma membrane (PM). When a vesicle fuses with the PM, the acidic vesicular lumen connects to the alkaline extracellular space and pHse fluorescence intensity increases (a, left). This is followed by a decrease in the pHse fluorescence intensity due to release of BDNF-pHse (a, middle). BDNF-pHse is released by full fusion events (a, middle and Fig. 5), as revealed by NH4Cl application at the end of experiments (a, right and Fig. 2). Acidic environment inside non-fusing vesicle quenches pHse fluorescence (b, left). The fluorescence intensity increases when the vesicle lumen is artificially alkalinized by NH4Cl (b, right). Endogenously expressed (pro)BDNF was omitted for clarity. Not drawn to scale (GIF 12 kb)
Rights and permissions
About this article
Cite this article
Stenovec, M., Lasič, E., Božić, M. et al. Ketamine Inhibits ATP-Evoked Exocytotic Release of Brain-Derived Neurotrophic Factor from Vesicles in Cultured Rat Astrocytes. Mol Neurobiol 53, 6882–6896 (2016). https://doi.org/10.1007/s12035-015-9562-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9562-y