Abstract
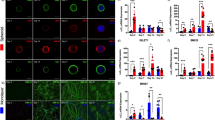
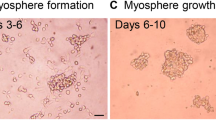
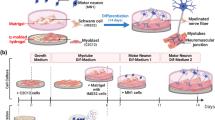
Dysfunction of the neuromuscular junction is involved in a wide range of muscular diseases. The development of neuromuscular junction through which skeletal muscle is innervated requires the functional modulation of acetylcholine receptor (AchR) clustering on myofibers. However, studies on AchR clustering in vitro are mostly done on monolayer muscle cell culture, which lacks a three-dimensional (3D) structure, a prominent limitation of the two-dimensional (2D) system. To enable a better understanding on the structure–function correlation underlying skeletal muscle innervation, a muscle system with a well-defined geometry mimicking the in vivo muscular setting is needed. Here, we report a 3D bio-artificial muscle (BAM) bioengineered from green fluorescent protein-transduced C3H murine myoblasts as a novel in vitro tissue-based model for muscle innervation studies. Our cell biological and molecular analysis showed that this BAM is structurally similar to in vivo muscle tissue and can reach the perinatal differentiation stage, higher than does 2D culture. Effective clustering and morphological maturation of AchRs on BAMs induced by agrin and laminin indicate the functional activity and plasticity of this BAM system toward innervation. Taken together, our results show that the BAM provides a favorable 3D environment that at least partially recapitulates real physiological skeletal muscle with regard to innervation. With a convenience of fabrication and manipulation, this 3D in vitro system offers a novel model for studying mechanisms underlying skeletal muscle innervation and testing therapeutic strategies for relevant nervous and muscular diseases.





Similar content being viewed by others
References
Sanes JR, Lichtman JW (2001) Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci 11:791–805
Hall ZW, Sanes JR (1993) Synaptic structure and development: the neuromuscular junction. Cell 72:99–121
Sanes JR, Lichtman JW (1999) Development of the vertebrate neuromuscular junction. Annu Rev Neurosci 22:389–442
Ferraro E, Molinari BL (2012) Molecular control of neuromuscular junction development. J Cachexia Sarcopenia Muscle 1:13–23
Shigemoto K, Kubo S, Mori S, Yamada S, Akiyoshi T, Miyazaki T (2010) Muscle weakness and neuromuscular junctions in aging and disease. Geriatr Gerontol Int 10:S137–147
Jang YC, Van Remmen H (2010) Age-associated alterations of the neuromuscular junction. Exp Gerontol 46(2–3):193–198
Balice-Gordon RJ (1997) Age-related changes in neuromuscular innervation. Muscle Nerve Suppl 5:S83–87
Engel AG, Sine SM (2005) Current understanding of congenital myasthenic syndromes. Curr Opin Pharmacol 3:308–321
Nogajski JH, Kiernan MC, Ouvrier RA, Andrews PI (2009) Congenital myasthenic syndromes. J Clin Neurosci 1:1–11
Conti-Fine BM, Milani M, Kaminski HJ (2006) Myasthenia gravis: past, present, and future. J Clin Invest 11:2843–2854
Arnold AS, Christe M, Handschin C, Handschin C (2012) A functional motor unit in the culture dish: co-culture of spinal cord explants and muscle cells. J Vis Exp 62. doi:10.3791/3616
Anderson MJ, Cohen MW (1977) Nerve-induced and spontaneous redistribution of acetylcholine receptors on cultured muscle cells. J Physiol 3:757–773
Bernareggi A, Luin E, Formaggio E, Fumagalli G, Lorenzon P (2012) Novel role for prepatterned nicotinic acetylcholine receptors during myogenesis. Muscle Nerve 1:112–121
Nelson CM, Bissell MJ (2006) Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol 22:287–309
Vandenburgh H, Shansky J, Benesch-Lee F, Skelly K, Spinazzola JM, Saponjian Y, Tseng BS (2009) Automated drug screening with contractile muscle tissue engineered from dystrophic myoblasts. FASEB J 10:3325–3334
Mueller-Klieser W (1997) Three-dimensional cell cultures: from molecular mechanisms to clinical applications. Am J Physiol 4(Pt 1):C1109–1123
Vandenburgh H, Shansky J, Benesch-Lee F, Barbata V, Reid J, Thorrez L, Valentini R, Crawford G (2008) Drug-screening platform based on the contractility of tissue-engineered muscle. Muscle Nerve 4:438–447
Bowe MA, Fallon JR (1995) The role of agrin in synapse formation. Annu Rev Neurosci 18:443–462
Kummer TT, Misgeld T, Lichtman JW, Sanes JR (2004) Nerve-independent formation of a topologically complex postsynaptic apparatus. J Cell Biol 7:1077–1087
Nishimune H, Valdez G, Jarad G, Moulson CL, Muller U, Miner JH, Sanes JR (2008) Laminins promote postsynaptic maturation by an autocrine mechanism at the neuromuscular junction. J Cell Biol 6:1201–1215
Thorrez L, Vandenburgh H, Callewaert N, Mertens N, Shansky J, Wang L, Arnout J, Collen D, Chuah M, Vandendriessche T (2006) Angiogenesis enhances factor IX delivery and persistence from retrievable human bioengineered muscle implants. Mol Ther 3:442–451
Vandenburgh H, Shansky J, Del Tatto M, Chromiak J (1999) Organogenesis of skeletal muscle in tissue culture. Methods Mol Med 18:217–225
Mahdavi V, Izumo S, Nadal-Ginard B (1987) Developmental and hormonal regulation of sarcomeric myosin heavy chain gene family. Circ Res 6:804–814
Wydro RM, Nguyen HT, Gubits RM, Nadal-Ginard B (1983) Characterization of sarcomeric myosin heavy chain genes. J Biol Chem 1:670–678
Lund AW, Yener B, Stegemann JP, Plopper GE (2009) The natural and engineered 3D microenvironment as a regulatory cue during stem cell fate determination. Tissue Eng Part B Rev 3:371–380
Nyga A, Cheema U, Loizidou M (2011) 3D tumour models: novel in vitro approaches to cancer studies. J Cell Commun Signal 3:239–248
Mazhar S, Herbst R (2012) The formation of complex acetylcholine receptor clusters requires MuSK kinase activity and structural information from the MuSK extracellular domain. Mol Cell Neurosci 4:475–486
Guo X, Gonzalez M, Stancescu M, Vandenburgh HH, Hickman JJ (2011) Neuromuscular junction formation between human stem cell-derived motoneurons and human skeletal muscle in a defined system. Biomaterials 36:9602–9611
Acknowledgments
We are grateful to Dr. Justin Fallon for providing mouse agrin. This research was supported by NIH grants R41 AR053386 and R43 AG029705. The authors confirm that no competing financial interests exist, and there has been no financial support for this research could have influenced its outcome.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, L., Shansky, J. & Vandenburgh, H. Induced Formation and Maturation of Acetylcholine Receptor Clusters in a Defined 3D Bio-Artificial Muscle. Mol Neurobiol 48, 397–403 (2013). https://doi.org/10.1007/s12035-013-8412-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8412-z