Abstract
The nerve growth factor (NGF)/TrkA-signaling is necessary for neural development, and its abnormality has been tightly associated with the tumorigenesis of various cancers originated from the nervous system. The characterization of key molecules involved in the NGF/TrkA-mediated neuronal differentiation could pave the way for the development of novel therapeutic strategies against neural malignancy. We have previously demonstrated that calreticulin (CRT) is a favorable prognostic factor highly expressed in primary neuroblastomas (NBs) with a more differentiated histology. In the present study, we found that the level of CRT was enhanced in NGF-stimulated differentiation of PC-12 cells through the extracellular signal-regulated kinase (ERK)-dependent mitogen-activated protein kinase (MAPK) pathway. A deficiency of CRT significantly decreased NGF-elicited neuronal differentiation. Furthermore, overexpression of CRT enhanced neuronal differentiation via simultaneously activating the ERK-dependent MAPK pathway. The Ca2+-regulating capacity of CRT was demonstrated to be indispensable for NGF-elicited neuronal differentiation. Intriguingly, the expression levels of CRT and NGF receptor TrkA were highly correlated in NBs with differentiated histology, and the coexistence of CRT and TrkA in NB tumors synergistically predicted a better 5-year survival rate. Together, our present findings delineate a CRT-dependent regulation of NGF-induced neuronal differentiation.





Similar content being viewed by others
References
Brodeur GM (2003) Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer 3(3):203–216
Brodeur GM, Minturn JE, Ho R, Simpson AM, Iyer R, Varela CR, Light JE, Kolla V, Evans AE (2009) Trk receptor expression and inhibition in neuroblastomas. Clin Cancer Res 15(10):3244–3250
Chang HH, Lee H, Hu MK, Tsao PN, Juan HF, Huang MC, Shih YY, Wang BJ, Jeng YM, Chang CL, Huang SF, Tsay YG, Hsieh FJ, Lin KH, Hsu WM, Liao YF (2010) Notch1 expression predicts an unfavorable prognosis and serves as a therapeutic target of patients with neuroblastoma. Clin Cancer Res 16(17):4411–4420
Chao MV (2003) Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 4(4):299–309
Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, Faldum A, Hero B, Iehara T, Machin D, Mosseri V, Simon T, Garaventa A, Castel V, Matthay KK (2009) The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol 27(2):289–297
Cowley S, Paterson H, Kemp P, Marshall CJ (1994) Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell 77(6):841–852
De Bernardi MA, Rabins SJ, Colangelo AM, Brooker G, Mocchetti I (1996) TrkA mediates the nerve growth factor-induced intracellular calcium accumulation. J Biol Chem 271(11):6092–6098
Fagan AM, Zhang H, Landis S, Smeyne RJ, Silos-Santiago I, Barbacid M (1996) TrkA, but not TrkC, receptors are essential for survival of sympathetic neurons in vivo. J Neurosci 16(19):6208–6218
Gelebart P, Opas M, Michalak M (2005) Calreticulin, a Ca2 + −binding chaperone of the endoplasmic reticulum. Int J Biochem Cell Biol 37(2):260–266
Guo L, Nakamura K, Lynch J, Opas M, Olson EN, Agellon LB, Michalak M (2002) Cardiac-specific expression of calcineurin reverses embryonic lethality in calreticulin-deficient mouse. J Biol Chem 277(52):50776–50779
Hsu WM, Hsieh FJ, Jeng YM, Kuo ML, Chen CN, Lai DM, Hsieh LJ, Wang BT, Tsao PN, Lee H, Lin MT, Lai HS, Chen WJ (2005) Calreticulin expression in neuroblastoma—a novel independent prognostic factor. Ann Oncol 16(2):314–321
Hsu WM, Lee H, Juan HF, Shih YY, Wang BJ, Pan CY, Jeng YM, Chang HH, Lu MY, Lin KH, Lai HS, Chen WJ, Tsay YG, Liao YF, Hsieh FJ (2008) Identification of GRP75 as an independent favorable prognostic marker of neuroblastoma by a proteomics analysis. Clin Cancer Res 14(19):6237–6245
Johnson S, Michalak M, Opas M, Eggleton P (2001) The ins and outs of calreticulin: from the ER lumen to the extracellular space. Trends Cell Biol 11(3):122–129
Mesaeli N, Nakamura K, Zvaritch E, Dickie P, Dziak E, Krause KH, Opas M, MacLennan DH, Michalak M (1999) Calreticulin is essential for cardiac development. J Cell Biol 144(5):857–868
Michalak M, Corbett EF, Mesaeli N, Nakamura K, Opas M (1999) Calreticulin: one protein, one gene, many functions. Biochem J 344(Pt 2):281–292
Nakagawara A (2004) Neural crest development and neuroblastoma: the genetic and biological link. Prog Brain Res 146:233–242
Nakagawara A, Ohira M (2004) Comprehensive genomics linking between neural development and cancer: neuroblastoma as a model. Cancer Lett 204(2):213–224
Nakagawara A, Arima-Nakagawara M, Scavarda NJ, Azar CG, Cantor AB, Brodeur GM (1993) Association between high levels of expression of the TRK gene and favorable outcome in human neuroblastoma. N Engl J Med 328(12):847–854
Pang L, Sawada T, Decker SJ, Saltiel AR (1995) Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. J Biol Chem 270(23):13585–13588
Peterson JR, Ora A, Van PN, Helenius A (1995) Transient, lectin-like association of calreticulin with folding intermediates of cellular and viral glycoproteins. Mol Biol Cell 6(9):1173–1184
Rauch F, Prud'homme J, Arabian A, Dedhar S, St-Arnaud R (2000) Heart, brain, and body wall defects in mice lacking calreticulin. Exp Cell Res 256(1):105–111
Shimada H (2003) The International Neuroblastoma Pathology Classification. Pathologica 95(5):240–241
Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B (1999a) Terminology and morphologic criteria of neuroblastic tumors: recommendations by the International Neuroblastoma Pathology Committee. Cancer 86(2):349–363
Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B, Stram DO, Gerbing RB, Lukens JN, Matthay KK, Castleberry RP (1999b) The International Neuroblastoma Pathology Classification (the Shimada system). Cancer 86(2):364–372
Tsien RY (1980) New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry 19(11):2396–2404
Tung YT, Hsu WM, Wang BJ, Wu SY, Yen CT, Hu MK, Liao YF (2008) Sodium selenite inhibits gamma-secretase activity through activation of ERK. Neurosci Lett 440(1):38–43
Van Hooff CO, Holthuis JC, Oestreicher AB, Boonstra J, De Graan PN, Gispen WH (1989) Nerve growth factor-induced changes in the intracellular localization of the protein kinase C substrate B-50 in pheochromocytoma PC12 cells. J Cell Biol 108(3):1115–1125
Acknowledgements
We thank the Core Facility of the Institute of Cellular and Organismic Biology, Academia Sinica, for technical support. The shRNA lentiviral vectors were obtained from the National RNAi Core Facility located at the Institute of Molecular Biology/Genomic Research Center, Academia Sinica, supported by the National Research Program for Genomic Medicine Grants of National Science Council, Taiwan (NSC 97-3112-B-001-016). This study was supported by the National Health Research Institutes (NHRI-EX96-9620NI to W.-M.H.), National Science Council, Taiwan (NSC 95-2314-B-002-155-MY2 to W.-M.H.), and Academia Sinica (to Y.-F.L.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
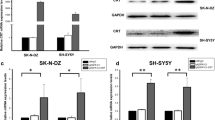
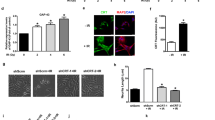
The effects of the ERK MAPK pathway on the expression of CRT and GAP43 in the absence of NGF. a PC-12 cells were treated with vehicle alone (0.1% DMSO), 20 μM U0126, or 20 μM PD98059 for 24 h. Cells were lysed and subjected to SDS–PAGE and Western blot analysis with the indicated antibodies. The levels of p-ERK, t-ERK, GAP43, CRT, and GAPDH (protein loading control) were quantitated by densitomtry. The relative levels of p-ERK/t-ERK, CRT (CRT versus GAPDH), and GAP43 (GAP43 versus GAPDH) were determined and compared with those of DMSO-treated cells that are referred to as 1-fold relative expression. *p < 0.05, compared to the DMSO-treated control. b PC-12 cells were transfected with the wild-type (WT) MEK1, a constitutively active MEK1 (MEK1-EE), a dominant-negative MEK1 (MEK-AA), or an empty vector (Mock) for 48 h. The expression levels of CRT were determined by SDS–PAGE and immunoblotting. The relative levels of p-ERK/t-ERK and CRT (CRT versus GAPDH) were determined and compared with those of Mock-transfected cells that are referred to as 1-fold relative expression. Data are shown as the mean (± SEM) of at least three independent experiments. *p < 0.05, compared to the mock-transfected control. The arrowhead indicates the endogenous expression of MEK1. c Cells transiently transfected with siRNA targeting ERK1/2 or scrambled sequences (NS) for 24 h. The relative levels of t-ERK (t-ERK versus GAPDH) and CRT (CRT versus GAPDH) were determined and compared with those of NS siRNA-transfected cells that are referred to as 1-fold relative expression. Results are presented as the mean (± SEM) of three independent experiments. *p < 0.05, compared to nonspecific siRNA (NS)-transfected control (DOCX 361 kb)
Fig. S2
The expression of GAP43 in PC-12 cells is not affected by cytosolic concentration of calcium in the absence of NGF. PC-12 cells were treated with 0.1% DMSO (control), BAPTA-AM (20 μM), or ionomycin (1 μM) for 24 h. Clarified lysates with equivalent amounts of proteins were analyzed by Western blotting. Normalized level of GAP43 in controls was referred to as 1-fold of relative expression. Data are shown as the mean (± SEM) of at least three independent experiments (DOC 113 kb)
Rights and permissions
About this article
Cite this article
Shih, YY., Nakagawara, A., Lee, H. et al. Calreticulin Mediates Nerve Growth Factor-Induced Neuronal Differentiation. J Mol Neurosci 47, 571–581 (2012). https://doi.org/10.1007/s12031-011-9683-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-011-9683-3