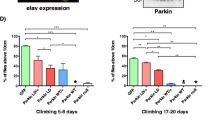
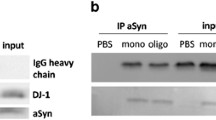
Abstract
Loss of functional Parkin is responsible for the death of midbrain dopaminergic neurons in human autosomal recessive juvenile parkinsonism. Since no cells express functional Parkin, it is unclear why other neuronal and non-neuronal populations are not also endangered. One possible explanation is that other neurons express a redundant ubiquitin–protein ligase (E3) that is absent from dopaminergic neurons. In this study, we demonstrate that human homolog of Drosophila ariadne-1 (HHARI) is a candidate for such a redundant function. In in vitro assays, HHARI binds to many of the same proteins as parkin, including CDCrel-1, synphilin-1, and CASK. In cell culture studies, HHARI forms aggresomes that are indistinguishable from those formed by parkin in terms of morphology, subcellular localization, incorporation of ubiquitin–proteasome components, and dependence on microtubules. In addition, endogenous HHARI is found in human Lewy bodies in both Parkinson’s disease and diffuse Lewy body disorder. Taken together, these data suggest that HHARI, and perhaps other Parkin-like E3 ligases, may serve redundant roles for parkin in different cell types.








Similar content being viewed by others
References
Aguilera M, Oliveros M, Martinez-Padron M, Barbas JA, Ferrus A (2000) Ariadne-1: a vital Drosophila gene is required in development and defines a new conserved family of ring-finger proteins. Genetics 155:1231–1244
Ardley HC, Tan NG, Rose SA, Markham AF, Robinson PA (2001) Features of the parkin/ariadne-like ubiquitin ligase, HHARI, that regulate its interaction with the ubiquitin-conjugating enzyme, Ubch7. J Biol Chem 276:19640–19647
Ardley HC, Scott GB, Rose SA, Tan NG, Markham AF, Robinson PA (2003) Inhibition of proteasomal activity causes inclusion formation in neuronal and non-neuronal cells overexpressing parkin. Mol Biol Cell 14:4541–4556
Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S (2004) Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 431:805–810
Bolhuis S, Richter-Landsberg C (2010) Effect of proteasome inhibition by MG-132 on HSP27 oligomerization, phosphorylation, and aggresome formation in the OLN-93 oligodendroglia cell line. J Neurochem 114:960–971
Cadena J (2009) The parkin-like ubiquitin E3 ligase ariadne-1 in the mammalian brain: potential implications for neurodegenerative disease. Ph.D. diss., University of Massachusetts Amherst, 2009. In: Dissertations & Theses @ University of Massachusetts @ Amherst [database on-line]; available from http://www.proquest.com (publication number AAT 3359131)
Capili AD, Edghill EL, Wu K, Borden KL (2004) Structure of the C-terminal RING finger from a RING-IBR-RING/TRIAD motif reveals a novel zinc-binding domain distinct from a RING. J Mol Biol 340:1117–1129
Choi P, Golts N, Snyder H et al (2001) Co-association of parkin and alpha-synuclein. Neuroreport 12:2839–2843
Chung KK, Zhang Y, Lim KL et al (2001) Parkin ubiquitinates the alpha-synuclein-interacting protein, synphilin-1: implications for Lewy-body formation in Parkinson disease. Nat Med 7:1144–1150
Chung KK, Thomas B, Li X et al (2004) S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science 304:1328–1331
Conway KA, Harper JD, Lansbury PT (1998) Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med 4:1318–1320
Cookson MR, Bandmann O (2010) Parkinson's disease: insights from pathways. Hum Mol Genet 15:R21–R27
Corboy MJ, Thomas PJ, Wigley WC (2005) Aggresome formation. Methods Mol Biol 301:305–327
Dawson TM, Dawson VL (2010) The role of parkin in familial and sporadic Parkinson's disease. Mov Disord 25(Suppl 1):S32–S39
Dehvari N, Sandebring A, Flores-Morales A et al (2009) Parkin-mediated ubiquitination regulates phospholipase C-gamma1. J Cell Mol Med 13:3061–3068
Dusonchet J, Bensadoun JC, Schneider BL, Aebischer P (2009) Targeted overexpression of the parkin substrate Pael-R in the nigrostriatal system of adult rats to model Parkinson's disease. Neurobiol Dis 35:32–41
Dziewulska D, Rafalowska J (2005) Proteinaceous intracellular inclusions in neurodegenerative disorders. Folia Neuropathol 43:51–63
Elbaz A, Moisan F (2009) Update in the epidemiology of Parkinson's disease. Curr Opin Neurol 4:454–460, 2008
Fallon L, Moreau F, Croft BG, Labib N, Gu WJ, Fon EA (2002) Parkin and CASK/LIN-2 associate via a PDZ-mediated interaction and are co-localized in lipid rafts and postsynaptic densities in brain. J Biol Chem 277:486–491
Forno LS (1996) Neuropathology of Parkinson's disease. J Neuropathol Exp Neurol 55:259–272
Fukae J, Sato S, Shiba K et al (2009) Programmed cell death-2 isoform1 is ubiquitinated by parkin and increased in the substantia nigra of patients with autosomal recessive Parkinson's disease. FEBS Lett 583:521–525
Garcia-Mata R, Bebok Z, Sorscher EJ, Sztul ES (1999) Characterization and dynamics of aggresome formation by a cytosolic GFP-chimera. J Cell Biol 146:1239–1254
Huang Z, de la Fuente-Fernandez R, Stoessl AJ (2003) Etiology of Parkinson's disease. Can J Neurol Sci 30(Suppl 1):S10–S18
Joch M, Ase AR, Chen CX et al (2007) Parkin-mediated monoubiquitination of the PDZ protein PICK1 regulates the activity of acid-sensing ion channels. Mol Biol Cell 18:3105–3118
Johnston JA, Ward CL, Kopito RR (1998) Aggresomes: a cellular response to misfolded proteins. J Cell Biol 143:1883–1898
Junn E, Lee SS, Suhr UT, Mouradian MM (2002) Parkin accumulation in aggresomes due to proteasome impairment. J Biol Chem 277:47870–47877
Kim SJ, Sung JY, Um JW et al (2003) Parkin cleaves intracellular alpha-synuclein inclusions via the activation of calpain. J Biol Chem 278:41890–41899
Kingsbury AE, Bandopadhyay R, Silveira-Moriyama L et al (2010) Brain stem pathology in Parkinson's disease: an evaluation of the Braak staging model. Mov Disord 25:2508–2515
Kitada T, Asakawa S, Hattori N et al (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392:605–608
Ko HS, von Coelln R, Sriram SR et al (2005) Accumulation of the authentic parkin substrate aminoacyl-tRNA synthetase cofactor, p38/JTV-1, leads to catecholaminergic cell death. J Neurosci 25:7968–7978
Kopito RR (2000) Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol 10:524–530
Lavoie MJ, Cortese GP, Ostaszewski BL, Schlossmacher MG (2007) The effects of oxidative stress on parkin and other E3 ligases. J Neurochem 103:2354–2368
Lesage S, Brice A (2009) Parkinson's disease: from monogenic forms to genetic susceptibility factors. Hum Mol Genet 18:R48–R59
Lim KL, Chew KC, Tan JM et al (2005) Parkin mediates nonclassical, proteasomal-independent ubiquitination of synphilin-1: implications for Lewy body formation. J Neurosci 25:2002–2009
Liu C, Fei E, Jia N et al (2007) Assembly of lysine 63-linked ubiquitin conjugates by phosphorylated alpha-synuclein implies Lewy body biogenesis. J Biol Chem 282:14558–14566
Marin I, Ferrus A (2002) Comparative genomics of the RBR family, including the Parkinson's disease-related gene parkin and the genes of the ariadne subfamily. Mol Biol Evol 19:2039–2050
McNaught KS, Shashidharan P, Perl DP, Jenner P, Olanow CW (2002) Aggresome-related biogenesis of Lewy bodies. Eur J Neurosci 16:2136–2148
Moynihan TP, Ardley HC, Nuber U et al (1999) The ubiquitin-conjugating enzymes UbcH7 and UbcH8 interact with RING finger/IBR motif-containing domains of HHARI and H7-AP1. J Biol Chem 274:30963–30968
Muqit MM, Davidson SM, Payne Smith MD et al (2004) Parkin is recruited into aggresomes in a stress-specific manner: over-expression of parkin reduces aggresome formation but can be dissociated from parkin's effect on neuronal survival. Hum Mol Genet 13:117–135
Okui M, Yamaki A, Takayanagi A, Kudoh J, Shimizu N, Shimizu Y (2005) Transcription factor single-minded 2 (SIM2) is ubiquitinated by the RING-IBR-RING-type E3 ubiquitin ligases. Exp Cell Res 309:220–228
Olanow CW, Perl DP, DeMartino GN, McNaught KS (2004) Lewy-body formation is an aggresome-related process: a hypothesis. Lancet Neurol 3:496–503
Olzmann JA, Chin LS (2008) Parkin-mediated K63-linked polyubiquitination: a signal for targeting misfolded proteins to the aggresome–autophagy pathway. Autophagy 4:85–87
Safadi SS, Shaw GS (2010) Differential interaction of the E3 ligase parkin with the proteasomal subunit S5a and the endocytic protein Eps15. J Biol Chem 285:1424–1434
Sakata E, Yamaguchi Y, Kurimoto E et al (2003) Parkin binds the Rpn10 subunit of 26S proteasomes through its ubiquitin-like domain. EMBO Rep 4:301–306
Savitt JM, Dawson VL, Dawson TM (2006) Diagnosis and treatment of Parkinson disease: molecules to medicine. J Clin Invest 116:1744–1754
Schlehe JS, Lutz AK, Pilsl A et al (2008) Aberrant folding of pathogenic parkin mutants: aggregation versus degradation. J Biol Chem 283:13771–13779
Schlossmacher MG, Frosch MP, Gai WP et al (2002) Parkin localizes to the Lewy bodies of Parkinson disease and dementia with Lewy bodies. Am J Pathol 160:1655–1667
Schwartz LM, Nambu JR, Wang Z (2002) Parkinsonism proteolysis and proteasomes. Cell Death Differ 9:479–482
Shults CW (2006) Lewy bodies. Proc Natl Acad Sci USA 103:1661–1668
Takahashi H, Ohama E, Suzuki S et al (1994) Familial juvenile parkinsonism: clinical and pathologic study in a family. Neurology 44:437–441
Takeda A, Hasegawa T, Matsuzaki-Kobayashi M et al (2006) Mechanisms of neuronal death in synucleinopathy. J Biomed Biotechnol 2006:19365
Tan NG, Ardley HC, Scott GB, Rose SA, Markham AF, Robinson PA (2003) Human homologue of ariadne promotes the ubiquitylation of translation initiation factor 4E homologous protein, 4EHP. FEBS Lett 554:501–504
Tanaka M, Kim YM, Lee G, Junn E, Iwatsubo T, Mouradian MM (2004) Aggresomes formed by alpha-synuclein and synphilin-1 are cytoprotective. J Biol Chem 279:4625–4631
Um JW, Chung KC (2006) Functional modulation of parkin through physical interaction with SUMO-1. J Neurosci Res 84:1543–1554
Wakabayashi K, Engelender S, Yoshimoto M, Tsuji S, Ross CA, Takahashi H (2000) Synphilin-1 is present in Lewy bodies in Parkinson's disease. Ann Neurol 47:521–523
Wang HQ, Imai Y, Inoue H et al (2008) Pael-R transgenic mice crossed with parkin deficient mice displayed progressive and selective catecholaminergic neuronal loss. J Neurochem 107:171–185
Wigley WC, Fabunmi RP, Lee MG et al (1999) Dynamic association of proteasomal machinery with the centrosome. J Cell Biol 145:481–490
Wong AW, Scales SJ, Reilly DE (2007a) DNA internalized via caveolae requires microtubule-dependent, Rab7-independent transport to the late endocytic pathway for delivery to the nucleus. J Biol Chem 282:22953–22963
Wong ES, Tan JM, Wang C et al (2007b) Relative sensitivity of parkin and other cysteine-containing enzymes to stress-induced solubility alterations. J Biol Chem 282:12310–12318
Yokochi M (1997) Familial juvenile parkinsonism. Eur Neurol 38(Suppl 1):29–33
Zhang Y, Gao J, Chung KK, Huang H, Dawson VL, Dawson TM (2000) Parkin functions as an E2-dependent ubiquitin- protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc Natl Acad Sci USA 97:13354–13359
We are sad to report that Dr. Juan G. Cadena passed away on July 29, 2010.
Acknowledgments
We thank Dr. Philip Robinson for the ariadne constructs and for helpful discussion; Dr. Ted Dawson for the Parkin constructs; Dr. David Sharlin for help with statistical analysis; Dr. Rolf Karlstrom for help with imaging; and Drs. Philip Robinson, Rod Murphey, and Patricia Wadsworth for a critical reading of the manuscript. This work was supported by grants from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parelkar, S.S., Cadena, J.G., Kim, C. et al. The Parkin-Like Human Homolog of Drosophila Ariadne-1 (HHARI) Can Induce Aggresome Formation in Mammalian Cells and Is Immunologically Detectable in Lewy Bodies. J Mol Neurosci 46, 109–121 (2012). https://doi.org/10.1007/s12031-011-9535-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-011-9535-1