Abstract
Background
This study assesses the utility of a hybrid optical instrument for noninvasive transcranial monitoring in the neurointensive care unit. The instrument is based on diffuse correlation spectroscopy (DCS) for measurement of cerebral blood flow (CBF), and near-infrared spectroscopy (NIRS) for measurement of oxy- and deoxy-hemoglobin concentration. DCS/NIRS measurements of CBF and oxygenation from frontal lobes are compared with concurrent xenon-enhanced computed tomography (XeCT) in patients during induced blood pressure changes and carbon dioxide arterial partial pressure variation.
Methods
Seven neurocritical care patients were included in the study. Relative CBF measured by DCS (rCBFDCS), and changes in oxy-hemoglobin (ΔHbO2), deoxy-hemoglobin (ΔHb), and total hemoglobin concentration (ΔTHC), measured by NIRS, were continuously monitored throughout XeCT during a baseline scan and a scan after intervention. CBF from XeCT regions-of-interest (ROIs) under the optical probes were used to calculate relative XeCT CBF (rCBFXeCT) and were then compared to rCBFDCS. Spearman’s rank coefficients were employed to test for associations between rCBFDCS and rCBFXeCT, as well as between rCBF from both modalities and NIRS parameters.
Results
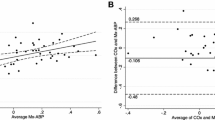
rCBFDCS and rCBFXeCT showed good correlation (r s = 0.73, P = 0.010) across the patient cohort. Moderate correlations between rCBFDCS and ΔHbO2/ΔTHC were also observed. Both NIRS and DCS distinguished the effects of xenon inhalation on CBF, which varied among the patients.
Conclusions
DCS measurements of CBF and NIRS measurements of tissue blood oxygenation were successfully obtained in neurocritical care patients. The potential for DCS to provide continuous, noninvasive bedside monitoring for the purpose of CBF management and individualized care is demonstrated.




Similar content being viewed by others
References
Waydhas C. Intrahospital transport of critically ill patients. Crit Care. 1999;3:R83–9.
Evans DH, McDicken WN. Doppler ultrasound: physics, instrumentation, and signal processing. 2nd ed. Chichester, NY: Wiley; 2000.
Vajkoczy P, Roth H, Horn P, et al. Continuous monitoring of regional cerebral blood flow: experimental and clinical validation of a novel thermal diffusion microprobe. J Neurosurg. 2000;93:265–74.
Kirkpatrick PJ, Smielewski P, Czosnyka M, Pickard JD. Continuous monitoring of cortical perfusion by laser Doppler flowmetry in ventilated patients with head injury. J Neurol Neurosurg Psychiatry. 1994;57:1382–8.
Reinhard M, Wehrle-Wieland E, Grabiak D, et al. Oscillatory cerebral hemodynamics—the macro- vs. microvascular level. J Neurol Sci. 2006;250:103–9.
Boas DA, Campbell LE, Yodh AG. Scattering and imaging with diffusing temporal field correlations. Phys Rev Lett. 1995;75:1855–8.
Maret G, Wolf PE. Multiple light-scattering from disordered media—the effect of Brownian-motion of scatterers. Z Phys B Con Mat. 1987;65:409–13.
Pine DJ, Weitz DA, Chaikin PM, Herbolzheimer E. Diffusing-wave spectroscopy. Phys Rev Lett. 1988;60:1134–7.
Villringer A, Chance B. Non-invasive optical spectroscopy and imaging of human brain function. Trends Neurosci. 1997;20:435–42.
Hillman EMC. Optical brain imaging in vivo: techniques and applications from animal to man. J Biomed Opt. 2007;12(5):051402-1–28.
Durduran T, Zhou C, Edlow BL, et al. Transcranial optical monitoring of cerebrovascular hemodynamics in acute stroke patients. Optics Express. 2009;17:3884–902.
Kim MN, Durduran T, Edlow BL, et al. Healthy aging alters the hemodynamic response to orthostatic stress. Stroke. 2008;39:715.
Zhou C, Eucker SA, Durduran T, et al. Diffuse optical monitoring of hemodynamic changes in piglet brain with closed head injury. J Biomed Opt. 2009;14(3):034015-1–11.
Pindzola RR, Yonas H. The xenon-enhanced computed tomography cerebral blood flow method. Neurosurgery. 1998;43:1488–92.
Yonas H, Pindzola RR, Johnson DW. Xenon/computed tomography cerebral blood flow and its use in clinical management. Neurosurg Clin N Am 1996;7:605.
Kety SS. The theory and applications of the exchange of inert gas at the lungs and tissues. Pharmacol Rev. 1951;3:1–41.
Durduran T, Yu GQ, Burnett MG, et al. Diffuse optical measurement of blood flow, blood oxygenation, and metabolism in a human brain during sensorimotor cortex activation. Optics Lett. 2004;29:1766–8.
Boas DA. Diffuse photon probes of structural and dynamical properties of turbid media: theory and biomedical applications. PhD thesis, University of Pennsylvania; 1996.
Duncan A, Meek JH, Clemence M, et al. Measurement of cranial optical path length as a function of age using phase resolved near infrared spectroscopy. Pediatr Res. 1996;39:889–94.
Boas DA, Yodh AG. Spatially varying dynamical properties of turbid media probed with diffusing temporal light correlation. J Opt Soc Am A. 1997;14:192–215.
Cheung C, Culver JP, Takahashi K, Greenberg JH, Yodh AG. In vivo cerebrovascular measurement combining diffuse near-infrared absorption and correlation spectroscopies. Phys Med Biol. 2001;46:2053–65.
Gur D, Yonas H, Good WF. Local cerebral blood flow by xenon-enhanced CT: current status, potential improvements, and future directions. Cerebrovasc Brain Metab Rev. 1989;1:68–86.
Obrist WD, Thompson HK, Wang HS, Wilkinson WE. Regional cerebral blood-flow estimated by xenon-133 inhalation. Stroke. 1975;6:245–56.
Meyer JS, Hayman LA, Yamamoto M, Sakai F, Nakajima S. Local cerebral blood-flow measured by Ct after stable xenon inhalation. Am J Roentgenol. 1980;135:239–51.
Zhang Z. Reliability and error analysis on xenon/CT CBF. Keio J Med. 2000;49(Suppl 1):A29–32.
Cullen SC, Gross EG. The anesthetic properties of xenon in animals and human beings, with additional observations on krypton. Science. 1951;113:580–2.
Horn P, Vajkoczy P, Thome C, Muench E, Schilling L, Schmiedek P. Xenon-induced flow activation in patients with cerebral insult who undergo xenon-enhanced CT blood flow studies. Am J Neuroradiol. 2001;22:1543–9.
Hartmann A, Dettmers C, Schuier FJ, Wassmann HD, Schumacher HW. Effect of stable xenon on regional cerebral blood-flow and the electroencephalogram in normal volunteers. Stroke. 1991;22:182–9.
Giller CA, Purdy P, Lindstrom WW. Effects of inhaled stable xenon on cerebral blood-flow velocity. Am J Neuroradiol. 1990;11:177–82.
Obrist WD, Zhang ZH, Yonas H. Effect of xenon-induced flow activation on xenon-enhanced computed tomography cerebral blood flow calculations. J Cereb Blood Flow Metab. 1998;18:1192–5.
Good WF, Gur D. Xenon-enhanced CT of the brain—effect of flow activation on derived cerebral blood-flow measurements. Am J Neuroradiol. 1991;12:83–5.
Sorensen AG. What is the meaning of quantitative CBF? Am J Neuroradiol. 2001;22:235–6.
Steiner LA, Andrews PJ. Monitoring the injured brain: ICP and CBF. Br J Anaesth. 2006;97:26–38.
Grandin CB, Duprez TP, Oppenheim C, Peeters A, Robert AR, Cosnard G. Which MR-derived perfusion parameters are the best predictors of infarct growth in hyperacute stroke? Comparative study between relative and quantitative measurements. Radiology. 2002;223:361–70.
Acknowledgments
We are grateful for the contributions of Mark Burnett who initiated the earliest protocols in the neurointensive care unit that led to this study. We also thank Dalton Hance, Justin Plaum, and neurointensive care unit nurses and radiology staff at HUP for technical assistance. Finally, we acknowledge helpful technical discussions with Mary Putt, Rickson Mesquita, and David Minkoff.
Disclosure/Disclaimer
Sources of Support (if applicable), name(s) of grantor(s), grant or contract numbers, name of author who received the funding, and specific material support given are as follows: National Institute of Heath: NS-054575 (MNK, JAD), NS-060653 (AGY), NS-045839 (JAD), HL-077699 (AGY), RR-02305 (AGY, JAD), and ED-26979 (TD). Thrasher Foundation: NR-0016 (TD).
Financial Disclosure
Patent pending.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, M.N., Durduran, T., Frangos, S. et al. Noninvasive Measurement of Cerebral Blood Flow and Blood Oxygenation Using Near-Infrared and Diffuse Correlation Spectroscopies in Critically Brain-Injured Adults. Neurocrit Care 12, 173–180 (2010). https://doi.org/10.1007/s12028-009-9305-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-009-9305-x