Abstract
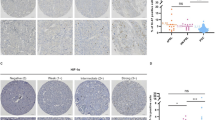
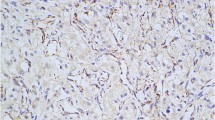
Pheochromocytomas are neuroendocrine tumors confined to the adrenal glands, and malignant pheochromocytomas can spread to various sites including the liver, lung, and bones. Paragangliomas occur in numerous locations in the body, so assessment of metastatic disease is more challenging, as patients with familial syndromes often have multiple, possibly independent paragangliomas. The most reliable criterion for malignancy in pheochromocytomas and paragangliomas is metastatic disease. Because there are few immunohistochemical markers that are useful in the diagnosis of malignancy in pheochromocytomas and paragangliomas before they metastasize, more markers are needed to characterize these tumors. Stathmin is a widely expressed 17-kDa cytoplasmic, microtubule destabilizing and sequestering phosphoprotein that is important in cell motility and cancer cell metastasis. It is upregulated in various malignancies. We examined stathmin expression in tissues from patients with pheochromocytomas (n = 48), malignant pheochromocytomas (n = 28), paragangliomas (n = 42), and malignant paragangliomas (n = 21) by immunohistochemistry using tissue microarrays (TMA) with a polyclonal antibody against stathmin. A series of other endocrine tissues and tumors (n = 70) were also examined for stathmin expression. Stathmin was more highly expressed in pheochromocytomas compared to normal adrenals, a finding confirmed by Western blot. There was higher expression of stathmin by immunohistochemical staining in malignant pheochromocytomas compared to pheochromocytomas without metastasis when analyzed by maximal staining (p = 0.012). Stathmin was present in a wide variety of endocrine tumors and was most highly expressed in rapidly proliferating tumors including anaplastic thyroid carcinomas, Merkel cell carcinomas of the skin and small cell carcinomas of the lung. These results show that stathmin is expressed at higher levels in more rapidly proliferating endocrine tumors. However, it is probably not useful as a stand-alone marker to determine malignancy in pheochromocytomas for individual tumors.


Similar content being viewed by others
References
Chrisoulidou A, Kaltsas G, Ilias I, et al. The diagnosis and management of malignant phaeochromocytoma and paragangliomas. Endocrine Relat Cancer. 14:569–85, 2007.
Brouwers FM, Eisenhofer G, Tao JJ, et al. High frequency of SDHB germline mutations in patients with malignant catecholamine-producing paragangliomas: Implications for genetic testing. J Clin Endocrinol Metab. 91:4505–9, 2006.
Neumann HPH, Pawlu C, Peczkowska M, et al. Distinct clinical features of paragangliomas syndromes associated with SDHB and SDHD gene mutations. JAMA. 292:943–51, 2004.
Madani R, Al-Hashmi M, Bliss R, et al. Ectopic pheochromocytoma: does the rule of tens apply? World J Surg. 31:849–54, 2007.
O’Riordain DS, Young WF, Grant CS, et al. Clinical spectrum and outcome of functional extraadrenal paragangliomas. World J Surg. 20:916–22, 1996.
Scott HW, Halter SA. Oncologic aspects of phaeochromocytoma: the importance of follow-up. Surgery. 96:1061–6, 1984.
Anouar Y, Yon L, Guillemot J, et al. Development of novel tools for the diagnosis and prognosis of pheochromocytoma using peptide marker immunoassay and gene expression profiling approaches. Ann N Y Acad Sci. 1073:533–40, 2006.
Brouwers FM, Elkahloun AG, Munson PJ, et al. Gene expression profiling of benign and malignant pheochromocytoma. Ann N Y Acad Sci. 1073:541–56, 2006.
Thouennon E, Elkahloun AG, Guillemot J, et al. Identification of potential gene markers and insights into the pathophysiology of pheochromocytoma malignancy. J Clin Endocrinol Metab. 92:4865–72, 2007.
Onda M, Emi M, Yoshida A, et al. Comprehensive gene expression profiling of anaplastic thyroid cancers with cDNA microarrays of 25,344 genes. Endocr Relat Cancer. 11:843–54, 2004.
Doye V, Bouttenin M-C, Sobel A. Phosphorylation of stathmin and other proteins related to nerve growth factor—induced regulation of PC12 cells. J Biol Chem. 265:11650–5, 1990.
Takekoshi K, Nomura F, Isobe K, et al. Identification of initial characterization of stathmin by the differential display method in nerve growth factor—treated PC 12 cells. Eur J Endocrinol. 138:707–12, 1998.
Beilharz EJ, Zhukovshy E, Lanahan AA, et al. Neuronal activity induction of the stathmin-like gene RB3 in the rat hippocampus: possible role in neuronal plasticity. J Neurosci. 18:9780–9, 1998.
Steinmetz MO. Structure and thermodynamics of the tubulin–stathmin interaction. J Struct Biol. 158:137–47, 2007.
Rubin CI, Atweh GF. The role of stathmin in the regulation of the cell cycle. J Cell Biochem. 93:242–50, 2004.
Iancu-Rubin C, Atweh GF. p27(Kip1) and stathmin share the stage for the first time. Trends Cell Biol. 15:346–8, 2005.
Cassimeris L. Regulation of microtubule dynamic instability. Cell Motil Cytoskelet. 26(4):275–81, 1993.
Nogales E. A structural view of microtubule dynamics. Cell Mol Life Sci. 56:133–42, 1999.
Cassimeris L. The oncoprotein 18/stathmin family of microtubule destabilizers. Curr Opin Cell Biol. 14:18–24, 2002.
Hanash S, Strahler JR, Kuick R, et al. Identification of a polypeptide associated with the malignant phenotype in acute leukemia. J Biol Chem. 263:12813–5, 1988.
Friedrich B, Gronberg H, Landstrom M, et al. Differentiation-stage specific expression of oncoprotein 18 in human and rat prostatic adenocarcinoma. Prostate. 27:102–9, 1995.
Golouh R, Cufer T, Sadikov A, et al. The prognostic value of Stathmin-1, S100A2, and SYK proteins in ER-positive primary breast cancer patients treated with adjuvant tamoxifen monotherapy: an immunohistochemical study. Breast Cancer Res Treat. DOI 10.1007/s10549-007-9724-3.
Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 4:844–7, 1998.
Rumilla KM, Erickson LA, Erickson AK, et al. Galectin-4 expression in carcinoid tumors. Endocr Pathol. 17:243–9, 2006.
Riss D, Jin L, Qian X, et al. Differential expression of galactin-3 in pituitary tumors. Cancer Res. 63:2251–8, 2003.
Lloyd RV, Jin L, Qian X, et al. Aberrant p27kip1 expression in endocrine and other tumors. Am J Pathol. 150:401–7, 1997.
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227:680–5, 1970.
DeLellis RA, Lloyd RV, Heitz PV, et al. Tumors of endocrine organs. World Health Organization Classification of Tumors. Lyon: IARC Press, 2004.
Lee S, Nakamura E, Yang H, et al. Neuronal apoptosis linked to EglN3 prolyl hydroxylase and familial pheochromocytoma genes: developmental culling and cancer. Cancer Cell. 8:155–67, 2005.
Perren A, Komminoth P. Familial pheochromocytomas and paragangliomas: stories from the sign-out room. Endocr Pathol. 17:337–44, 2006.
Tischler AS. Molecular and cellular biology of pheochromocytomas and extra-adrenal paragangliomas. Endocr Pathol. 17:321–8, 2006.
Benn DE, Gimenez-Roqueplo AP, Reilly JR, et al. Clinical presentation and penetrance of pheochromocytoma/paraganglioma syndromes. J Clin Endocrinol Metab. 91:827–36, 2006.
Klein RD, Jin L, Rumilla K, et al. Germline SDHB mutations are common in patients with apparently sporadic sympathetic paragangliomas. Diagn Mol Pathol. 2008, March 28 [Epub ahead of print].
Baldassarre G, Belletti B, Nocoloso MS, et al. p27(Kip1)–stathmin interaction influences sarcoma cell migration and invasion. Cancer Cells. 7:51–63, 2005.
Garrett S, Kapoor TM. Microtubule assembly: catastrophe factors to the rescue. Cancer Biol. 13:R910–2, 2003.
Niethammer P, Bastiaens P, Karsenti E. Stathmin–tubulin interaction gradients in motile and mitotic cells. Science. 303:1862–6, 2004.
McAllister SS, Becker-Hapak M, Pintucci G, et al. Novel p27(kip1) C-terminal scatter domain mediates Rac-dependent cell migration independent of cell cycle arrest functions. Mol Cell Biol. 23:216–28, 2003.
Acknowledgment
Thanks to Christine Lohse for help with statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: R. V. Lloyd MD, PhD
Rights and permissions
About this article
Cite this article
Sadow, P.M., Rumilla, K.M., Erickson, L.A. et al. Stathmin Expression in Pheochromocytomas, Paragangliomas, and in other Endocrine Tumors. Endocr Pathol 19, 97–103 (2008). https://doi.org/10.1007/s12022-008-9028-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-008-9028-0