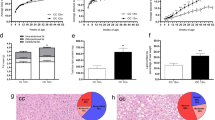
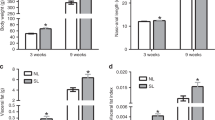
Abstract
High-calorie diet (HCD) feeding in mice predisposes offspring for impaired glucose homeostasis and obesity. However, the mechanisms underlying these detrimental effects of maternal nutrition, especially during early life of offspring, are incompletely understood. MicroRNAs (miRNAs) are small non-coding RNAs that can regulate target gene expression. Here we hypothesized that impaired metabolic health in offspring from HCD-fed dams at weaning is associated with dysregulated expression of hepatic miRNAs. Dams were fed a chow diet (CD; 11.4 % kcal fat, 62.8 % from carbohydrate, 25.8 % from protein) or HCD (58 % kcal from fat; 25.6 % from carbohydrate, 16.4 % from protein) during gestation and lactation, and metabolic health was assessed in male offspring at weaning. Hepatic levels of miRNAs and target genes were investigated in offspring from CD- or HCD-fed dams using gene and protein expression. Maternal HCD feeding impaired metabolic health in offspring compared to offspring from CD-fed dams. Microarray analysis indicated that expressions of miR-615-5p, miR-3079-5p, miR-124*, and miR-101b* were downregulated, whereas miR-143* was upregulated, in livers from offspring from HCD-fed dams. Our functional enrichment analysis indicated that the target genes of these differentially expressed miRNAs, including tumor necrosis factor-α (TNF-α) and mitogen-activated protein kinase 1 (MAPK1), were mapped to inflammatory pathways. Finally, we verified that both mRNA and protein levels of the pro-inflammatory modulators TNF-α and MAPK1 were significantly increased in livers of offspring from HCD-fed dams at weaning. Maternal HCD feeding predisposes offspring to a higher body weight and impaired glucose metabolism at weaning. To the best of knowledge, our study is the first to show that maternal HCD consumption impairs metabolic health, modulates hepatic miRNA expression, and increases markers of hepatic inflammation in offspring as early as at weaning age.




Similar content being viewed by others
References
M. Slatkin, Epigenetic inheritance and the missing heritability problem. Genetics 182(3), 845–850 (2009). doi:10.1534/genetics.109.102798
G.C. Curhan, W.C. Willett, E.B. Rimm, D. Spiegelman, A.L. Ascherio, M.J. Stampfer, Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation 94(12), 3246–3250 (1996)
D. Gniuli, A. Calcagno, M.E. Caristo, A. Mancuso, V. Macchi, G. Mingrone, R. Vettor, Effects of high-fat diet exposure during fetal life on type 2 diabetes development in the progeny. J. Lipid Res. 49(9), 1936–1945 (2008). doi:10.1194/jlr.M800033-JLR200
H.S. Lee, Impact of maternal diet on the epigenome during in utero life and the developmental programming of diseases in childhood and adulthood. Nutrients 7(11), 9492–9507 (2015). doi:10.3390/nu7115467
O. Dumortier, C. Hinault, N. Gautier, S. Patouraux, V. Casamento, E. Van Obberghen, Maternal protein restriction leads to pancreatic failure in offspring: role of misexpressed microRNA-375. Diabetes (2014). doi:10.2337/db13-1431
S. Lie, J.L. Morrison, O. Williams-Wyss, C.M. Suter, D.T. Humphreys, S.E. Ozanne, S. Zhang, S.M. Maclaughlin, D.O. Kleemann, S.K. Walker, C.T. Roberts, I.C. McMillen, Periconceptional undernutrition programs changes in insulin-signaling molecules and microRNAs in skeletal muscle in singleton and twin fetal sheep. Biol. Reprod. 90(1), 5 (2014). doi:10.1095/biolreprod.113.109751
S. Lie, J.L. Morrison, O. Williams-Wyss, C.M. Suter, D.T. Humphreys, S.E. Ozanne, S. Zhang, S.M. MacLaughlin, D.O. Kleemann, S.K. Walker, C.T. Roberts, I.C. McMillen, Impact of embryo number and maternal undernutrition around the time of conception on insulin signaling and gluconeogenic factors and microRNAs in the liver of fetal sheep. Am. j. Physiol. Endocrinol. Metab. 306(9), E1013–E1024 (2014). doi:10.1152/ajpendo.00553.2013
M.A. Reddy, R. Natarajan, Epigenetic mechanisms in diabetic vascular complications. Cardiovasc. Res. 90(3), 421–429 (2011). doi:10.1093/cvr/cvr024
O. Aguilera, A.F. Fernandez, A. Munoz, M.F. Fraga, Epigenetics and environment: a complex relationship. J. Appl. Physiol. 109(1), 243–251 (2010). doi:10.1152/japplphysiol.00068.2010
J.A. Deiuliis, MicroRNAs as regulators of metabolic disease: pathophysiologic significance and emerging role as biomarkers and therapeutics. Int. J. Obes. 40(1), 88–101 (2005). doi:10.1038/ijo.2015.170
V. Rottiers, A.M. Naar, MicroRNAs in metabolism and metabolic disorders. Nat. Rev. Mol. Cell Biol. 13(4), 239–250 (2012). doi:10.1038/nrm3313
S.D. Jordan, M. Kruger, D.M. Willmes, N. Redemann, F.T. Wunderlich, H.S. Bronneke, C. Merkwirth, H. Kashkar, V.M. Olkkonen, T. Bottger, T. Braun, J. Seibler, J.C. Bruning, Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism. Nat. Cell Biol. 13(4), 434–446 (2011). doi:10.1038/ncb2211
J.W. Kornfeld, C. Baitzel, A.C. Konner, H.T. Nicholls, M.C. Vogt, K. Herrmanns, L. Scheja, C. Haumaitre, A.M. Wolf, U. Knippschild, J. Seibler, S. Cereghini, J. Heeren, M. Stoffel, J.C. Bruning, Obesity-induced overexpression of miR-802 impairs glucose metabolism through silencing of Hnf1b. Nature 494(7435), 111–115 (2013). doi:10.1038/nature11793
T. Pentinat, M. Ramon-Krauel, J. Cebria, R. Diaz, J.C. Jimenez-Chillaron, Transgenerational inheritance of glucose intolerance in a mouse model of neonatal overnutrition. Endocrinology 151(12), 5617–5623 (2010). doi:10.1210/en.2010-0684
L. Williams, Y. Seki, P.M. Vuguin, M.J. Charron, Animal models of in utero exposure to a high fat diet: a review. Biochim. Biophys. Acta 1842(3), 507–519 (2014). doi:10.1016/j.bbadis.2013.07.006
M.E. Symonds, S.P. Sebert, M.A. Hyatt, H. Budge, Nutritional programming of the metabolic syndrome. Nat. Rev. Endocrinol. 5(11), 604–610 (2009). doi:10.1038/nrendo.2009.195
P.M. Miotto, L.M. Castelli, F. Amoye, P.J. LeBlanc, S.J. Peters, B.D. Roy, W.E. Ward, Maternal high fat feeding does not have long-lasting effects on body composition and bone health in female and male Wistar rat offspring at young adulthood. Molecules 18(12), 15094–15109 (2013). doi:10.3390/molecules181215094
J.A. McKay, L. Xie, C. Manus, S.A. Langie, R.J. Maxwell, D. Ford, J.C. Mathers, Metabolic effects of a high-fat diet post-weaning after low maternal dietary folate during pregnancy and lactation. Mol. Nutr. Food Res. (2014). doi:10.1002/mnfr.201300615
K.D. Bruce, F.R. Cagampang, M. Argenton, J. Zhang, P.L. Ethirajan, G.C. Burdge, A.C. Bateman, G.F. Clough, L. Poston, M.A. Hanson, J.M. McConnell, C.D. Byrne, Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology 50(6), 1796–1808 (2009). doi:10.1002/hep.23205
H. Jiang, W. Wu, M. Zhang, J. Li, Y. Peng, T.T. Miao, H. Zhu, G. Xu, Aberrant upregulation of miR-21 in placental tissues of macrosomia. J. Perinatol. 34(9), 658–663 (2014). doi:10.1038/jp.2014.58
J. Zhang, F. Zhang, X. Didelot, K.D. Bruce, F.R. Cagampang, M. Vatish, M. Hanson, H. Lehnert, A. Ceriello, C.D. Byrne, Maternal high fat diet during pregnancy and lactation alters hepatic expression of insulin like growth factor-2 and key microRNAs in the adult offspring. BMC Genom. 10, 478 (2009). doi:10.1186/1471-2164-10-478
R.O. Benatti, A.M. Melo, F.O. Borges, L.M. Ignacio-Souza, L.A. Simino, M. Milanski, L.A. Velloso, M.A. Torsoni, A.S. Torsoni, Maternal high-fat diet consumption modulates hepatic lipid metabolism and microRNA-122 (miR-122) and microRNA-370 (miR-370) expression in offspring. Br. J. Nutr. 111(12), 2112–2122 (2014). doi:10.1017/s0007114514000579
J. Zheng, X. Xiao, Q. Zhang, M. Yu, J. Xu, Z. Wang, C. Qi, T. Wang, Maternal and post-weaning high-fat, high-sucrose diet modulates glucose homeostasis and hypothalamic POMC promoter methylation in mouse offspring. Metab. Brain Dis. 30(5), 1129–1137 (2015). doi:10.1007/s11011-015-9678-9
M.S. Winzell, B. Ahren, The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 53(Suppl 3), S215–S219 (2004)
Y. Luo, C. Zhang, F. Tang, J. Zhao, C. Shen, C. Wang, P. Yu, M. Wang, Y. Li, J.I. Di, R. Chen, G. Rili, Bioinformatics identification of potentially involved microRNAs in Tibetan with gastric cancer based on microRNA profiling. Cancer Cell Int. 15, 115 (2015). doi:10.1186/s12935-015-0266-1
H. Dweep, N. Gretz, miRWalk2.0: a comprehensive atlas of microRNA-target interactions. Nat. Methods 12(8), 697 (2015). doi:10.1038/nmeth.3485
Q. Zhang, X. Xiao, M. Li, W. Li, M. Yu, H. Zhang, Z. Wang, H. Xiang, Acarbose reduces blood glucose by activating miR-10a-5p and miR-664 in diabetic rats. PLoS One 8(11), e79697 (2013). doi:10.1371/journal.pone.0079697
G. Verdile, K.N. Keane, V.F. Cruzat, S. Medic, M. Sabale, J. Rowles, N. Wijesekara, R.N. Martins, P.E. Fraser, P. Newsholme, Inflammation and oxidative stress: the molecular connectivity between insulin resistance, obesity, and Alzheimer’s disease. Mediat. Inflamm. 2015, 105828 (2015). doi:10.1155/2015/105828
H. Chen, Cellular inflammatory responses: novel insights for obesity and insulin resistance. Pharmacol. Res. 53(6), 469–477 (2006). doi:10.1016/j.phrs.2006.03.003
V.T. Samuel, G.I. Shulman, The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J. Clin. Investig. 126(1), 12–22 (2016). doi:10.1172/jci77812
L. Ferrero-Miliani, O.H. Nielsen, P.S. Andersen, S.E. Girardin, Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1beta generation. Clin. Exp. Immunol. 147(2), 227–235 (2007). doi:10.1111/j.1365-2249.2006.03261.x
J.L. Saben, E.S. Bales, M.R. Jackman, D. Orlicky, P.S. MacLean, J.L. McManaman, Maternal obesity reduces milk lipid production in lactating mice by inhibiting acetyl-CoA carboxylase and impairing fatty acid synthesis. PLoS ONE 9(5), e98066 (2014). doi:10.1371/journal.pone.0098066
C.J. Bautista, S. Montano, V. Ramirez, A. Morales, P.W. Nathanielsz, N.A. Bobadilla, E. Zambrano, Changes in milk composition in obese rats consuming a high-fat diet. Br. J. Nutr. 115(3), 538–546 (2016). doi:10.1017/s0007114515004547
M.C. Vogt, L. Paeger, S. Hess, S.M. Steculorum, M. Awazawa, B. Hampel, S. Neupert, H.T. Nicholls, J. Mauer, A.C. Hausen, R. Predel, P. Kloppenburg, T.L. Horvath, J.C. Bruning, Neonatal insulin action impairs hypothalamic neurocircuit formation in response to maternal high-fat feeding. Cell 156(3), 495–509 (2014). doi:10.1016/j.cell.2014.01.008
N.G. Ashino, K.N. Saito, F.D. Souza, F.S. Nakutz, E.A. Roman, L.A. Velloso, A.S. Torsoni, M.A. Torsoni, Maternal high-fat feeding through pregnancy and lactation predisposes mouse offspring to molecular insulin resistance and fatty liver. J. Nutr. Biochem. 23(4), 341–348 (2012). doi:10.1016/j.jnutbio.2010.12.011
M.G. Pruis, A. Lendvai, V.W. Bloks, M.V. Zwier, J.F. Baller, A. de Bruin, A.K. Groen, T. Plosch, Maternal western diet primes non-alcoholic fatty liver disease in adult mouse offspring. Acta Physiologica 210(1), 215–227 (2014). doi:10.1111/apha.12197
L. Backdahl, A. Bushell, S. Beck, Inflammatory signalling as mediator of epigenetic modulation in tissue-specific chronic inflammation. Int. J. Biochem. Cell Biol. 41(1), 176–184 (2009). doi:10.1016/j.biocel.2008.08.023
D. Zhou, Y.X. Pan, Pathophysiological basis for compromised health beyond generations: role of maternal high-fat diet and low-grade chronic inflammation. J. Nutr. Biochem. 26(1), 1–8 (2015). doi:10.1016/j.jnutbio.2014.06.011
J.M. Kyriakis, J. Avruch, Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81(2), 807–869 (2001)
J.L. Marques-Rocha, M. Samblas, F.I. Milagro, J. Bressan, J.A. Martinez, A. Marti, Noncoding RNAs, cytokines, and inflammation-related diseases. FASEB J. 29(9), 3595–3611 (2015). doi:10.1096/fj.14-260323
N. Zhang, J. Lei, H. Lei, X. Ruan, Q. Liu, Y. Chen, W. Huang, MicroRNA-101 overexpression by IL-6 and TNF-alpha inhibits cholesterol efflux by suppressing ATP-binding cassette transporter A1 expression. Exp. Cell Res. 336(1), 33–42 (2015). doi:10.1016/j.yexcr.2015.05.023
E. Tsitsiou, M.A. Lindsay, microRNAs and the immune response. Curr. Opin. Pharmacol. 9(4), 514–520 (2009). doi:10.1016/j.coph.2009.05.003
E.M. Munch, R.A. Harris, M. Mohammad, A.L. Benham, S.M. Pejerrey, L. Showalter, M. Hu, C.D. Shope, P.D. Maningat, P.H. Gunaratne, M. Haymond, K. Aagaard, Transcriptome profiling of microRNA by Next-Gen deep sequencing reveals known and novel miRNA species in the lipid fraction of human breast milk. PLoS One 8(2), e50564 (2013). doi:10.1371/journal.pone.0050564
P. Casas-Agustench, F.S. Fernandes, M.G.T. do Carmo, F. Visioli, E. Herrera, A. Davalos, Consumption of distinct dietary lipids during early pregnancy differentially modulates the expression of microRNAs in mothers and offspring. PLoS One 10(2), e0117858 (2015). doi:10.1371/journal.pone.0117858
P. Casas-Agustench, E. Iglesias-Gutierrez, A. Davalos, Mother’s nutritional miRNA legacy: nutrition during pregnancy and its possible implications to develop cardiometabolic disease in later life. Pharmacol. Res. 100, 322–334 (2015). doi:10.1016/j.phrs.2015.08.017
I.D. Kilic, Y. Dodurga, B. Uludag, Y.I. Alihanoglu, B.S. Yildiz, Y. Enli, M. Secme, H.E. Bostanci, MicroRNA -143 and -223 in obesity. Gene 560(2), 140–142 (2015). doi:10.1016/j.gene.2015.01.048
Y. Teng, R. Zhang, C. Liu, L. Zhou, H. Wang, W. Zhuang, Y. Huang, Z. Hong, miR-143 inhibits interleukin-13-induced inflammatory cytokine and mucus production in nasal epithelial cells from allergic rhinitis patients by targeting IL13Ralpha1. Biochem. Biophys. Res. Commun. 457(1), 58–64 (2015). doi:10.1016/j.bbrc.2014.12.058
D. Li, Z. Lu, J. Jia, Z. Zheng, S. Lin, MiR-124 is related to podocytic adhesive capacity damage in STZ-induced uninephrectomized diabetic rats. Kidney Blood Press. Res. 37(4–5), 422–431 (2013). doi:10.1159/000355721
J.H. Wu, Y. Gao, A.J. Ren, S.H. Zhao, M. Zhong, Y.J. Peng, W. Shen, M. Jing, L. Liu, Altered microRNA expression profiles in retinas with diabetic retinopathy. Ophthalmic Res. 47(4), 195–201 (2012). doi:10.1159/000331992
Y. Miyamoto, A.S. Mauer, S. Kumar, J.L. Mott, H. Malhi, Mmu-miR-615-3p regulates lipoapoptosis by inhibiting C/EBP homologous protein. PLoS One 9(10), e109637 (2014). doi:10.1371/journal.pone.0109637
P. Siengdee, N. Trakooljul, E. Murani, M. Schwerin, K. Wimmers, S. Ponsuksili, MicroRNAs regulate cellular ATP levels by targeting mitochondrial energy metabolism genes during C2C12 myoblast differentiation. PLoS One 10(5), e0127850 (2015). doi:10.1371/journal.pone.0127850
Acknowledgments
This project was supported by the National Natural Science Foundation of China (No. 81570715 and No. 81170736) and the National Natural Science Foundation for Young Scholars of China (No. 81300649), the National Key Program of Clinical Science and Peking Union Medical College Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zheng, J., Zhang, Q., Mul, J.D. et al. Maternal high-calorie diet is associated with altered hepatic microRNA expression and impaired metabolic health in offspring at weaning age. Endocrine 54, 70–80 (2016). https://doi.org/10.1007/s12020-016-0959-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-0959-9