Abstract
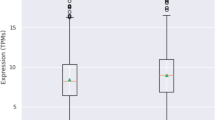
Parkinson’s disease (PD) is a progressive neurodegenerative disease with complex etiology. Both genetic and environmental factors play significant role. Apart from candidate genes, some modifier genes have been reported to be associated with the altered risk of PD. Previous studies have identified Apolipoprotein E (APOE), Cathepsin D (CTSD), and Brain-Derived Neurotrophic Factor (BDNF) as key players of neurodegenerative pathways with their variants associated with different neurodegenerative diseases. Hence, this study aims to identify the potential role of these modifier genes in the pathogenesis of PD among Eastern Indian PD patients. A case–control study was performed using 302 clinically diagnosed PD patients and 304 ethnically matched controls. Promoter SNPs of APOE (rs449647, rs405509) and BDNF (rs56164415), and coding SNPs of APOE (rs429358, rs7412 resulting in ε2, ε3, and ε4 alleles), CTSD (rs17571), and BDNF (rs6265) were analyzed by PCR–RFLP and bidirectional sequencing. The effect of rs56164415 on BDNF expression was characterized by Luciferase assay. APOEε4 allele was significantly overrepresented (p value = 0.0003) among PD patients, whereas ε3 allele was predominant in the control population. The promoter haplotype (A-rs449647, G-rs405509) of APOE was preponderant among female PD patients posing risk. No association was found for CTSD polymorphism. The ‘T/T’ genotype of BDNF rs56164415 was overrepresented (p-value = 0.02) among early onset PD patients. Expression of BDNF for the ‘T/T’ variant was significantly lower (p-value = 0.012) than the ‘C/C’ variant, suggesting a possible role in PD pathogenesis. This study suggests that APOE and BDNF may serve as modifier loci among eastern Indian PD patients.

Similar content being viewed by others
References
Biswas, A., Gupta, A., Naiya, T., Das, G., Neogi, R., Datta, S., et al. (2006). Molecular pathogenesis of Parkinson’s disease: Identification of mutations in the Parkin gene in Indian patients. Parkinsonism & Related Disorders, 12(7), 420–426.
Biswas, A., Sadhukhan, T., Majumder, S., Misra, A. K., Das, S. K., Ray, I. G. K., et al. (2010). Evaluation of PINK1 variants in Indian Parkinson’s disease patients. Parkinsonism & Related Disorders, 16(3), 167–171.
Bras, J., Guerreiro, R., & Hardy, J. (2015). SnapShot: Genetics of Parkinson’s disease. Cell, 160(3), 570. e1.
Chen, H. K., Ji, Z. S., Dodson, S. E., Miranda, R. D., Rosenblum, C. I., Reynolds, I. J., et al. (2011). Apolipoprotein E4 domain interaction mediates detrimental effects on mitochondria and is a potential therapeutic target for Alzheimer disease. The Journal of Biological Chemistry, 286(7), 5215–5221.
Currie, L. J., Harrison, M. B., Trugman, J. M., Bennett, J. P., & Wooten, G. F. (2004). Postmenopausal estrogen use affects risk for Parkinson disease. Archives of Neurology, 61(6), 886–888.
Dai, L., Wang, D., Meng, H., Zhang, K., Fu, L., Wu, Y., et al. (2013a). Association between the BDNF G196A and C270T polymorphisms and Parkinson’s disease: A meta-analysis. International Journal of Neuroscience, 123(10), 675–683.
Dai, L., Wang, D., Meng, H., Zhang, K., Fu, L., Wu, Y., et al. (2013b). Association between the BDNF G196A and C270T polymorphisms and Parkinson’s disease: A meta-analysis. The International Journal of Neuroscience, 123(10), 675–683.
Das, G., Misra, A. K., Das, S. K., Ray, K., & Ray, J. (2009). Microtubule-associated protein tau (MAPT) influences the risk of Parkinson’s disease among Indians. Neuroscience Letters, 460(1), 16–20.
Das, G., Misra, A. K., Das, S. K., Ray, K., & Ray, J. (2012). Role of tau kinases (CDK5R1 and GSK3B) in Parkinson’s disease: a study from India. Neurobiology of Aging, 33(7), 1485. e9–e15.
Davidson, Y., Gibbons, L., Pritchard, A., Hardicre, J., Wren, J., Tian, J., et al. (2006). Genetic associations between cathepsin D exon 2 C– > T polymorphism and Alzheimer’s disease, and pathological correlations with genotype. Journal of Neurology, Neurosurgery and Psychiatry, 77(4), 515–517.
Deng, H., Wang, P., & Jankovic, J. (2018). The genetics of Parkinson disease. Ageing Research Reviews, 42, 72–85.
Egan, M. F., Kojima, M., Callicott, J. H., Goldberg, T. E., Kolachana, B. S., Bertolino, A., et al. (2003). The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell, 112(2), 257–269.
Elliott, D. A., Weickert, C. S., & Garner, B. (2010). Apolipoproteins in the brain: Implications for neurological and psychiatric disorders. Clinical Lipidology, 51(4), 555–573.
Hauser, P. S., Narayanaswami, V., & Ryan, R. O. (2011). Apolipoprotein E: From lipid transport to neurobiology. Progress in Lipid Research, 50(1), 62–74.
Howells, D. W., Porritt, M. J., Wong, J. Y., Batchelor, P. E., Kalnins, R., Hughes, A. J., et al. (2000). Reduced BDNF mRNA expression in the Parkinson’s disease substantia nigra. Experimental Neurology, 166(1), 127–135.
Hudry, E., Klickstein, J., Cannavo, C., Jackson, R., Muzikansky, A., Gandhi, S., et al. (2019). Opposing roles of apolipoprotein E in aging and neurodegeneration. Life Science Alliance, 2(1), 1–16.
Karakasis, C., Kalinderi, K., Katsarou, Z., Fidani, L., & Bostantjopoulou, S. (2011). Association of brain-derived neurotrophic factor (BDNF) Val66Met polymorphism with Parkinson’s disease in a Greek population. Journal of Clinical Neuroscience: Official Journal of the Neurosurgical Society of Australasia, 18(12), 1744–1745.
Lambert, J. C., Coyle, N., & Lendon, C. (2004). The allelic modulation of apolipoprotein E expression by oestrogen: Potential relevance for Alzheimer’s disease. Journal of Medical Genetics, 41(2), 104–112.
Larsen, S. B., Hanss, Z., & Kruger, R. (2018). The genetic architecture of mitochondrial dysfunction in Parkinson’s disease. Cell and Tissue Research, 373, 21–37.
Lee, Y. H., & Song, G. G. (2014). BDNF 196 G/A and 270 C/T polymorphisms and susceptibility to Parkinson’s disease: A meta-analysis. Journal of Motor Behavior, 46(1), 59–66.
Li, J., Luo, J., Liu, L., Fu, H., & Tang, L. (2018). The genetic association between apolipoprotein E gene polymorphism and Parkinson disease: A meta-Analysis of 47 studies. Medicine, 97(43), 1–12.
Liu, C. C., Liu, C. C., Kanekiyo, T., Xu, H., & Bu, G. (2013). Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nature Reviews. Neurology, 9(2), 106–118.
Maloney, B., Ge, Y. W., Petersen, R. C., Hardy, J., Rogers, J. T., Perez-Tur, J., et al. (2010). Functional characterization of three single-nucleotide polymorphisms present in the human APOE promoter sequence: Differential effects in neuronal cells and on DNA-protein interactions, American journal of medical genetics. Part B, Neuropsychiatric genetics: The Official Publication of the International Society of Psychiatric Genetics, 153B(1), 185–201.
Mariga, A., Mitre, M., & Chao, M. V. (2017). Consequences of brain-derived neurotrophic factor withdrawal in CNS neurons and implications in disease. Neurobiology of Disease, 97(Pt B), 73–79.
Nakamura, T., Watanabe, A., Fujino, T., Hosono, T., & Michikawa, M. (2009). Apolipoprotein E4 (1-272) fragment is associated with mitochondrial proteins and affects mitochondrial function in neuronal cells. Molecular Neurodegeneration, 4, 35.
Ntais, C., Polycarpou, A., & Ioannidis, J. P. (2004). Meta-analysis of the association of the cathepsin D Ala224Val gene polymorphism with the risk of Alzheimer’s disease: A HuGE gene-disease association review. American Journal of Epidemiology, 159(6), 527–536.
Parain, K., Murer, M. G., Yan, Q., Faucheux, B., Agid, Y., Hirsch, E., et al. (1999). Reduced expression of brain-derived neurotrophic factor protein in Parkinson’s disease substantia nigra. NeuroReport, 10(3), 557–561.
Pruunsild, P., Kazantseva, A., Aid, T., Palm, K., & Timmusk, T. (2007). Dissecting the human BDNF locus: Bidirectional transcription, complex splicing, and multiple promoters. Genomics, 90(3), 397–406.
Ragonese, P., D’Amelio, M., Salemi, G., Aridon, P., Gammino, M., Epifanio, A., et al. (2004). Risk of Parkinson disease in women: Effect of reproductive characteristics. Neurology, 62(11), 2010–2014.
Saarela, M. S., Lehtimaki, T., Rinne, J. O., Huhtala, H., Rontu, R., Hervonen, A., et al. (2006). No association between the brain-derived neurotrophic factor 196 G > A or 270 C > T polymorphisms and Alzheimer’s or Parkinson’s disease. Folia Neuropathologica/Association of Polish Neuropathologists and Medical Research Centre, Polish Academy of Sciences, 44(1), 12–16.
Sadhukhan, T., Biswas, A., Das, S. K., Ray, K., & Ray, J. (2012). DJ-1 variants in Indian Parkinson’s disease patients. Disease Markers, 33(3), 127–135.
Sadhukhan, D., Das, G., Biswas, A., Ghosh, S., Das, S. K., Ray, K., et al. (2018). Evaluation of FGF 20 variants for susceptibility to Parkinson’s disease in Eastern Indians. Neuroscience Letters, 675, 68–73.
Schulte, T., Bohringer, S., Schols, L., Muller, T., Fischer, C., Riess, O., et al. (2003). Modulation of disease risk according to a cathepsin D/apolipoprotein E genotype in Parkinson’s disease. Journal of Neural Transmission, 110(7), 749–755.
Singh, N. K., Banerjee, B. D., Bala, K., Basu, M., Dung Dung, A. A., & Chhillar, N. (2014). APOE and LRPAP1 gene polymorphism and risk of Parkinson’s disease. Neurological Sciences, 35(7), 1075–1081.
Zhou, W., Scott, S. A., Shelton, S. B., & Crutcher, K. A. (2006). Cathepsin D-mediated proteolysis of apolipoprotein E: Possible role in Alzheimer’s disease. Neuroscience, 143(3), 689–701.
Zintzaras, E., & Hadjigeorgiou, G. M. (2005). The role of G196A polymorphism in the brain-derived neurotrophic factor gene in the cause of Parkinson’s disease: A meta-analysis. Journal of Human Genetics, 50(11), 560–566.
Acknowledgements
The authors thank all the subjects who participated in this study and Shubhrajit Roy and Kaustav Das Gupta for helpful suggestion towards the manuscript preparation. This work has been supported by the funding from Department of Science & Technology, Cognitive Science Research Initiative (DST-CSRI), Govt. of India. PP was supported by University Research Fellowship, TS and SB was supported by fellowships from the Govt. of West Bengal. AB was supported by DST-CSRI (SR/CSI/PDF-32/2014). SC was supported by DS Kothari Post-Doctoral Fellowship, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pal, P., Sadhukhan, T., Chakraborty, S. et al. Role of Apolipoprotein E, Cathepsin D, and Brain-Derived Neurotrophic Factor in Parkinson’s Disease: A Study from Eastern India. Neuromol Med 21, 287–294 (2019). https://doi.org/10.1007/s12017-019-08548-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-019-08548-4