Abstract
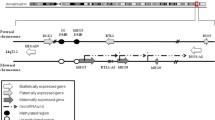
The paternally-imprinted genes insulin-like growth factor 2 (IGF2), H19, delta-like homologue 1 (DLK1), and maternally-expressed gene 3 (MEG3) are expressed from the tandem gene loci IGF2–H19 and DLK1–MEG3, which play crucial roles in initiating embryogenesis and development. The erasure of imprinting (EOI) at differentially methylated regions (DMRs) which regulate the expression of these genes maintains the developmental quiescence of primordial germ cells (PGCs) migrating through the embryo proper during embryogenesis and prevents them from forming teratomas. To address the potential involvement of the IGF2–H19 and DLK1–MEG3 loci in the pathogenesis of embryonal carcinoma (EC), we investigated their genomic imprinting at DMRs in the human PGC-derived EC cell line NTera-2 (NT2). We observed EOI at the IGF2–H19 locus and, somewhat to our surprise, a loss of imprinting (LOI) at the DLK1–MEG3 locus. As a result, NT2 cells express imprinted gene ratios from these loci such that there are i) low levels of the proliferation-promoting IGF2 relative to ii) high levels of the proliferation-inhibiting long noncoding RNA (lncRNA) H19 and iii) high levels of proliferation-promoting DLK1 relative to iv) low levels of the proliferation-inhibiting lncRNA MEG3. Consistent with this pattern of expression, the knockdown of DLK1 mRNA by shRNA resulted in decreased in vitro cell proliferation and in vivo tumor growth as well as decreased in vivo organ seeding by NT2 cells. Furthermore, treatment of NT2 cells with the DNA methyltransferase inhibitor 5-aza-2′-deoxycytidine (5-azaD) inhibited their proliferation. This inhibition was accompanied by changes in expression of both tandem gene sets: a decrease in the expression of DLK1 and upregulation of the proliferation-inhibiting lncRNA MEG3, and at the same time upregulation of IGF2 and downregulation of the lncRNA H19. These results suggest that the DLK1–MEG3 locus, and not the IGF2–H19 locus, drives the tumorigenicity of NT2 cells. Based on these results, we identified DLK1 as a novel treatment target for EC that could be downregulated by 5-azaD.





Similar content being viewed by others
References
Nogales, F. F., Dulcey, I., & Preda, O. (2014). Germ cell tumors of the ovary: An update. Archives of Pathology and Laboratory Medicine, 138(3), 351–362.
Mostofi, F. K., Sesterhenn, I. A., & Davis Jr., C. J. (1988). Developments in histopathology of testicular germ cell tumors. Seminars in Urology, 6(3), 171–188.
Krag, J. G., Barlebo, H., Olsen, J., et al. (1984). Testicular germ cell tumours in Denmark 1976-1980. Pathology of 1058 consecutive cases. Acta Radiologica. Oncology, 23(4), 239–247.
Kawakami, T., Zhang, C., Okada, Y., & Okamoto, K. (2006). Erasure of methylation imprint at the promoter and CTCF-binding site upstream of H19 in human testicular germ cell tumors of adolescents indicate their fetal germ cell origin. Oncogene, 25(23), 3225–3236.
Sievers, S., Alemazkour, K., Zahn, S., et al. (2005). IGF2/H19 imprinting analysis of human germ cell tumors (GCTs) using the methylation-sensitive single-nucleotide primer extension method reflects the origin of GCTs in different stages of primordial germ cell development. Genes. Chromosomes and Cancer, 44(3), 256–264.
Irie, N., Tang, W. W., & Azim, S. M. (2014). Germ cell specification and pluripotency in mammals: A perspective from early embryogenesis. Reproductive Medicine and Biology, 13(4), 203–215.
Andrews, P. W., Damjanov, I., Simon, D., et al. (1984). Pluripotent embryonal carcinoma clones derived from the human teratocarcinoma cell line Tera-2. Differentiation in vivo and in vitro. Laboratory Investigation, 50(2), 147–162.
Andrews, P. W., Matin, M. M., Bahrami, A. R., Damjanov, I., Gokhale, P., & Draper, J. S. (2005). Embryonic stem (ES) cells and embryonal carcinoma (EC) cells: Opposite sides of the same coin. Biochemical Society Transactions, 33(Pt 6), 1526–1530.
Kleinsmith, L. J., & Pierce Jr., G. B. (1964). Multipotentiality of single embryonal carcinoma cells. Cancer Research, 24(9), 1544–1551.
Li, J. Y., Lees-Murdock, D. J., Xu, G. L., & Walsh, C. P. (2004). Timing of establishment of paternal methylation imprints in the mouse. Genomics, 84(6), 952–960.
Keniry, A., Oxley, D., Monnier, P., et al. (2012). The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r. Nature Cell Biology, 14(7), 659–665.
Qian, P., He, X. C., Paulson, A., et al. (2016). The Dlk1-Gtl2 locus preserves LT-HSC function by inhibiting the PI3K-mTOR pathway to restrict mitochondrial metabolism. Cell Stem Cell, 18(2), 214–228.
Saitou, M., & Yamaji, M. (2012). Primordial germ cells in mice. Cold Spring Harbor Perspectives in Biology, 4(11), 1–19.
Kono, T., Obata, Y., Wu, Q., et al. (2004). Birth of parthenogenetic mice that can develop to adulthood. Nature, 428(6985), 860–864.
Wu, Q., Kumagai, T., Kawahara, M., et al. (2006). Regulated expression of two sets of paternally imprinted genes is necessary for mouse parthenogenetic development to term. Reproduction, 131(3), 481–488.
Anderson, J., Gordon, A., McManus, A., Shipley, J., & Pritchard-Jones, K. (1999). Disruption of imprinted genes at chromosome region 11p15.5 in paediatric rhabdomyosarcoma. Neoplasia, 1(4), 340–348.
Steenman, M. J., Rainier, S., Dobry, C. J., Grundy, P., Horon, I. L., & Feinberg, A. P. (1994). Loss of imprinting of IGF2 is linked to reduced expression and abnormal methylation of H19 in Wilms' tumour. Nature Genetics, 7(3), 433–439.
Reik W., Brown K. W., Schneid H., Le Bouc Y., Bickmore W., Maher E. R. (1995). Imprinting mutations in the Beckwith-Wiedemann syndrome suggested by altered imprinting pattern in the IGF2-H19 domain. Human Molecular Genetics, 4(12), 2379–2385.
Schneider, G., Bowser, M. J., Shin, D. M., Barr, F. G., & Ratajczak, M. Z. (2014). The paternally imprinted DLK1-GTL2 locus is differentially methylated in embryonal and alveolar rhabdomyosarcomas. International Journal of Oncology, 44(1), 295–300.
Tarnowski, M., Tkacz, M., Czerewaty, M., Poniewierska-Baran, A., Grymula, K., & Ratajczak, M. Z. (2015). 5Azacytidine inhibits human rhabdomyosarcoma cell growth by downregulating insulinlike growth factor 2 expression and reactivating the H19 gene product miR675, which negatively affects insulinlike growth factors and insulin signaling. International Journal of Oncology, 46(5), 2241–2250.
Biswal, B. K., Beyrouthy, M. J., Hever-Jardine, M. P., et al. (2012). Acute hypersensitivity of pluripotent testicular cancer-derived embryonal carcinoma to low-dose 5-aza deoxycytidine is associated with global DNA damage-associated p53 activation, anti-pluripotency and DNA demethylation. PLoS One, 7(12), e53003.
Wongtrakoongate, P., Li, J., & Andrews, P. W. (2014). Aza-deoxycytidine induces apoptosis or differentiation via DNMT3B and targets embryonal carcinoma cells but not their differentiated derivatives. British Journal of Cancer, 110(8), 2131–2138.
Kim, Y. (2010). Effect of retinoic acid and delta-like 1 homologue (DLK1) on differentiation in neuroblastoma. Nutrition Research and Practice, 4(4), 276–282.
Gomes, C., Smith, S. C., Youssef, M. N., Zheng, J. J., Hagg, T., & Hetman, M. (2011). RNA polymerase 1-driven transcription as a mediator of BDNF-induced neurite outgrowth. Journal of Biological Chemistry, 286(6), 4357–4363.
Sellers, Z. P., Schneider, G., Bujko, K., Suszynska, M., & Pedziwiatr, D. (2017). Do Cancer cell lines have fixed or fluctuating stem cell phenotypes? - studies with the NTera2 cell line. Stem Cell Reviews and Reports, 13(5), 603–610.
Liu, Y., Shin, S., Zeng, X., et al. (2006). Genome wide profiling of human embryonic stem cells (hESCs), their derivatives and embryonal carcinoma cells to develop base profiles of U.S. federal government approved hESC lines. BMC Developmental Biology, 6(20), 1–16.
Ratajczak, M. Z., Ratajczak, J., Suszynska, M., Miller, D. M., Kucia, M., & Shin, D. M. (2017). A novel view of the adult stem cell compartment from the perspective of a quiescent population of very small embryonic-like stem cells. Circulation Research, 120(1), 166–178.
Shin, D. M., Zuba-Surma, E. K., Wu, W., et al. (2009). Novel epigenetic mechanisms that control pluripotency and quiescence of adult bone marrow-derived Oct4(+) very small embryonic-like stem cells. Leukemia, 23(11), 2042–2051.
Venkatraman, A., He, X. C., Thorvaldsen, J. L., et al. (2013). Maternal imprinting at the H19-Igf2 locus maintains adult haematopoietic stem cell quiescence. Nature, 500(7462), 345–349.
Lin, S. P., Youngson, N., Takada, S., et al. (2003). Asymmetric regulation of imprinting on the maternal and paternal chromosomes at the Dlk1-Gtl2 imprinted cluster on mouse chromosome 12. Nature Genetics, 35(1), 97–102.
Baker, J., Liu, J. P., Robertson, E. J., & Efstratiadis, A. (1993). Role of insulin-like growth factors in embryonic and postnatal growth. Cell, 75(1), 73–82.
Stadtfeld, M., Apostolou, E., Akutsu, H., et al. (2010). Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature, 465(7295), 175–181.
Van Limpt V. A., Chan A. J., Van Sluis P. G., Caron H. N., Van Noesel C. J., Versteeg R. (2003). High delta-like 1 expression in a subset of neuroblastoma cell lines corresponds to a differentiated chromaffin cell type. International Journal of Cancer, 105(1), 61–69.
Liu, Y., Tan, J., Li, L., et al. (2010). [study on the molecular mechanisms of dlk1 stimulated lung cancer cell proliferation]. Zhongguo Fei Ai Za Zhi. Chinese Journal of Lung Cancer, 13(10), 923–927.
Huang, J., Zhang, X., Zhang, M., et al. (2007). Up-regulation of DLK1 as an imprinted gene could contribute to human hepatocellular carcinoma. Carcinogenesis, 28(5), 1094–1103.
Sakajiri, S., O'Kelly, J., Yin, D., et al. (2005). Dlk1 in normal and abnormal hematopoiesis. Leukemia, 19(8), 1404–1410.
Kim, Y., Lin, Q., Zelterman, D., & Yun, Z. (2009). Hypoxia-regulated delta-like 1 homologue enhances cancer cell stemness and tumorigenicity. Cancer Research, 69(24), 9271–9280.
Li, L., Tan, J., Zhang, Y., et al. (2014). DLK1 promotes lung cancer cell invasion through upregulation of MMP9 expression depending on notch signaling. PLoS One, 9(3), e91509.
Bujak, E., Ritz, D., & Neri, D. (2015). A monoclonal antibody to human DLK1 reveals differential expression in Cancer and absence in healthy tissues. Antibodies, 4(2), 71–87.
Fukuzawa, R., Heathcott, R. W., Morison, I. M., & Reeve, A. E. (2005). Imprinting, expression, and localisation of DLK1 in Wilms tumours. Journal of Clinical Pathology, 58(2), 145–150.
Ansell, P. J., Zhou, Y., Schjeide, B. M., et al. (2007). Regulation of growth hormone expression by Delta-like protein 1 (Dlk1). Molecular and Cellular Endocrinology, 271(1–2), 55–63.
Begum, A., Kim, Y., Lin, Q., & Yun, Z. (2012). DLK1, delta-like 1 homolog (drosophila), regulates tumor cell differentiation in vivo. Cancer Letters, 318(1), 26–33.
Li, L., Forman, S. J., & Bhatia, R. (2005). Expression of DLK1 in hematopoietic cells results in inhibition of differentiation and proliferation. Oncogene, 24(27), 4472–4476.
Begum, A., Lin, Q., Yu, C., Kim, Y., & Yun, Z. (2014). Interaction of delta-like 1 homolog (drosophila) with prohibitins and its impact on tumor cell clonogenicity. Molecular Cancer Research, 12(1), 155–164.
Peng, F., Wang, J. H., Fan, W. J., et al. (2017). Glycolysis gatekeeper PDK1 reprograms breast cancer stem cells under hypoxia. Oncogene, 37(8), 1062–1074.
Zeira, E., Abramovitch, R., Meir, K., et al. (2015). The knockdown of H19lncRNA reveals its regulatory role in pluripotency and tumorigenesis of human embryonic carcinoma cells. Oncotarget, 6(33), 34691–34703.
Hayashi, Y., Otsuka, K., Ebina, M., et al. (2017). Distinct requirements for energy metabolism in mouse primordial germ cells and their reprogramming to embryonic germ cells. Proceedings of the National Academy of Sciences of the United States of America, 114(31), 8289–8294.
Rugg-Gunn, P. J., Ferguson-Smith, A. C., & Pedersen, R. A. (2005). Epigenetic status of human embryonic stem cells. Nature Genetics, 37(6), 585–587.
Xie, P., Sun, Y., Ouyang, Q., et al. (2014). Physiological oxygen prevents frequent silencing of the DLK1-DIO3 cluster during human embryonic stem cells culture. Stem Cells, 32(2), 391–401.
Enver, T., Soneji, S., Joshi, C., et al. (2005). Cellular differentiation hierarchies in normal and culture-adapted human embryonic stem cells. Human Molecular Genetics, 14(21), 3129–3140.
Bhartiya, D. (2017). Pluripotent stem cells in adult tissues: Struggling to be acknowledged over two decades. Stem Cell Reviews and Reports, 13(6), 713–724.
Smadja, D. M. (2017). Bone marrow very small embryonic-like stem cells: New generation of autologous cell therapy soon ready for prime time? Stem Cell Reviews and Reports, 13(2), 198–201.
Ratajczak, M. Z., Bartke, A., & Darzynkiewicz, Z. (2017). Prolonged growth hormone/insulin/insulin-like growth factor nutrient response signaling pathway as a silent killer of stem cells and a culprit in aging. Stem Cell Reviews and Reports, 13(4), 443–453.
Acknowledgements
This work was supported by NIH grants 2R01 DK074720 and R01HL112788, the Stella and Henry Endowment, and the OPUS grant DEC-2016/23/B/NZ3/03157 to MZR.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplementary Figure 1
Methylation of DMRs within maternally-imprinted loci as assessed by COBRA. (PPTX 4004 kb)
Rights and permissions
About this article
Cite this article
Sellers, Z.P., Schneider, G., Maj, M. et al. Analysis of the Paternally-Imprinted DLK1–MEG3 and IGF2–H19 Tandem Gene Loci in NT2 Embryonal Carcinoma Cells Identifies DLK1 as a Potential Therapeutic Target. Stem Cell Rev and Rep 14, 823–836 (2018). https://doi.org/10.1007/s12015-018-9838-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-018-9838-5