Abstract
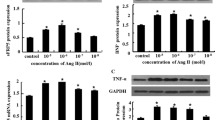
Sunitinib (SUN) is a multi-targeted tyrosine kinase inhibitor used for the treatment of gastrointestinal stromal tumors and renal cell carcinoma. Cardiotoxicity has been reported as a significant side effect associated with the SUN treatment, yet the mechanism is poorly understood. The main purpose of this study was to investigate the potential effects of SUN on cardiac hypertrophic genes and the role of mitogen-activated protein kinases (MAPKs) signaling pathway in rat cardiomyocyte H9c2 cell line. In the present study, real-time quantitative polymerase chain reaction showed that the treatment of H9c2 cells with increasing concentrations of SUN (0, 1, 2.5, and 5 µM) significantly induced hypertrophic gene markers, such as brain natriuretic peptides (BNP) and myosin heavy chain (β-MHC and α-MHC) in concentration- and time-dependent manners. The onset of mRNA induction was observed as early as 9 h and remained elevated for at least 18 h after treatment with SUN 5 µM. At the protein level, Western blot analysis showed that SUN increased BNP and β-MHC, while it inhibited α-MHC protein levels in a concentration-dependent manner. These SUN-mediated effects were associated with increase in cell size and hypertrophy by approximately 70 % at the highest concentration, 5 µM. Importantly, inhibition of the MAPK signaling pathway using SB203580 (p38 MAPK inhibitor), U0126 (extracellular signal-regulated kinase inhibitor), and SP600125 (c-Jun NH2-terminal kinase inhibitor) significantly potentiated the SUN-induced BNP and β-MHC mRNA levels, but did alter the α-MHC level. Whereas at the protein level, MAPK inhibitors generally decreased the SUN-induced BNP, whereas only SB and U0 increased β-MHC protein levels with no effect on α-MHC, which were associated with a significant decrease in cell size. Together, these results indicate that SUN induced hypertrophic gene expression through MAPK-dependent mechanisms.
Access this article
We’re sorry, something doesn't seem to be working properly.
Please try refreshing the page. If that doesn't work, please contact support so we can address the problem.







Similar content being viewed by others
References
Atkins, M., Jones, C. A., & Kirkpatrick, P. (2006). Sunitinib maleate. Nature Reviews Drug Discovery, 5, 279–280.
Faivre, S., Demetri, G., Sargent, W., & Raymond, E. (2007). Molecular basis for sunitinib efficacy and future clinical development. Nature Reviews Drug Discovery, 6, 734–745.
Kassem, M. G., Motiur Rahman, A. F., & Korashy, H. M. (2012). Sunitinib malate. Profiles of Drug Substances, Excipients, and Related Methodology, 37, 363–388.
Rini, B. I. (2007). Sunitinib. Expert Opinion on Pharmacotherapy, 8, 2359–2369.
Cheng, A. L., Kang, Y. K., Lin, D. Y., Park, J. W., Kudo, M., Qin, S., et al. (2013). Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. Journal of Clinical Oncology, 31, 4067–4075.
Waqar, S. N., Gopalan, P. K., Williams, K., Devarakonda, S., & Govindan, R. (2013). A phase I trial of sunitinib and rapamycin in patients with advanced non-small cell lung cancer. Chemotherapy, 59, 8–13.
Goodman, V. L., Rock, E. P., Dagher, R., Ramchandani, R. P., Abraham, S., Gobburu, J. V., et al. (2007). Approval summary: Sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clinical Cancer Research, 13, 1367–1373.
Chu, T. F., Rupnick, M. A., Kerkela, R., Dallabrida, S. M., Zurakowski, D., Nguyen, L., et al. (2007). Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet, 370, 2011–2019.
Imig, J. D., Zhao, X., Capdevila, J. H., Morisseau, C., & Hammock, B. D. (2002). Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension, 39, 690–694.
Force, T., Krause, D. S., & Van Etten, R. A. (2007). Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nature Reviews Cancer, 7, 332–344.
Gustafsson, A. B., & Gottlieb, R. A. (2007). Bcl-2 family members and apoptosis, taken to heart. American Journal of Physiology Cell Physiology, 292, C45–C51.
Hasinoff, B. B., Patel, D., & O’Hara, K. A. (2008). Mechanisms of myocyte cytotoxicity induced by the multiple receptor tyrosine kinase inhibitor sunitinib. Molecular Pharmacology, 74, 1722–1728.
Pearson, G., Robinson, F., Beers Gibson, T., Xu, B. E., Karandikar, M., Berman, K., et al. (2001). Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocrine Reviews, 22, 153–183.
Liang, Q., & Molkentin, J. D. (2003). Redefining the roles of p38 and JNK signaling in cardiac hypertrophy: dichotomy between cultured myocytes and animal models. Journal of Molecular and Cellular Cardiology, 35, 1385–1394.
Yin, H., Zhang, J., Lin, H., Wang, R., Qiao, Y., Wang, B., et al. (2008). p38 mitogen-activated protein kinase inhibition decreases TNFalpha secretion and protects against left ventricular remodeling in rats with myocardial ischemia. Inflammation, 31, 65–73.
Zhang, W., Elimban, V., Nijjar, M. S., Gupta, S. K., & Dhalla, N. S. (2003). Role of mitogen-activated protein kinase in cardiac hypertrophy and heart failure. Experimental and Clinical Cardiology, 8, 173–183.
Barry, S. P., Davidson, S. M., & Townsend, P. A. (2008). Molecular regulation of cardiac hypertrophy. International Journal of Biochemistry and Cell Biology, 40, 2023–2039.
Lin, H., Xu, L., Liu, H., Sun, Q., Chen, Z., & Yuan, G. (2011). KLF4 promotes the odontoblastic differentiation of human dental pulp cells. Journal of Endodontics, 37, 948–954.
Chang, S. W., Lee, S. Y., Kum, K. Y., & Kim, E. C. (2014). Effects of ProRoot MTA, Bioaggregate, and Micromega MTA on odontoblastic differentiation in human dental pulp cells. Journal of Endodontics, 40, 113–118.
Kerkela, R., Ilves, M., Pikkarainen, S., Tokola, H., Ronkainen, V. P., Majalahti, T., et al. (2011). Key roles of endothelin-1 and p38 MAPK in the regulation of atrial stretch response. American Journal of Physiology Regulatory, Integrative and Comparative Physiology, 300, R140–R149.
Kerkela, R., Pikkarainen, S., Majalahti-Palviainen, T., Tokola, H., & Ruskoaho, H. (2002). Distinct roles of mitogen-activated protein kinase pathways in GATA-4 transcription factor-mediated regulation of B-type natriuretic peptide gene. Journal of Biological Chemistry, 277, 13752–13760.
Han, J., & Molkentin, J. D. (2000). Regulation of MEF2 by p38 MAPK and its implication in cardiomyocyte biology. Trends in Cardiovascular Medicine, 10, 19–22.
Yang, C. C., Ornatsky, O. I., McDermott, J. C., Cruz, T. F., & Prody, C. A. (1998). Interaction of myocyte enhancer factor 2 (MEF2) with a mitogen-activated protein kinase, ERK5/BMK1. Nucleic Acids Research, 26, 4771–4777.
Liang, Q., Wiese, R. J., Bueno, O. F., Dai, Y. S., Markham, B. E., & Molkentin, J. D. (2001). The transcription factor GATA4 is activated by extracellular signal-regulated kinase 1- and 2-mediated phosphorylation of serine 105 in cardiomyocytes. Molecular and Cellular Biology, 21, 7460–7469.
Korashy, H. M., & El-Kadi, A. O. (2008). Modulation of TCDD-mediated induction of cytochrome P450 1A1 by mercury, lead, and copper in human HepG2 cell line. Toxicology In Vitro, 22, 154–158.
Korashy, H. M., Maayah, Z. H., Abd-Allah, A. R., El-Kadi, A. O., & Alhaider, A. A. (2012). Camel milk triggers apoptotic signaling pathways in human hepatoma HepG2 and breast cancer MCF7 cell lines through transcriptional mechanism. Journal of biomedicine and biotechnology, 2012, 593195.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods, 25, 402–408.
Korashy, H. M., & El-Kadi, A. O. (2004). Differential effects of mercury, lead and copper on the constitutive and inducible expression of aryl hydrocarbon receptor (AHR)-regulated genes in cultured hepatoma Hepa 1c1c7 cells. Toxicology, 201, 153–172.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Sambrook, J., Fritsch, E. F., & Maniatatis, T. (1989). In N. Ford (Ed.), Molecular cloning: A laboratory manual. Plainview, NY: Cold Spring Harbour Laboratory Press.
Korashy, H. M., & El-Kadi, A. O. (2006). The role of aryl hydrocarbon receptor and the reactive oxygen species in the modulation of glutathione transferase by heavy metals in murine hepatoma cell lines. Chemico-Biological Interactions, 162, 237–248.
Korashy, H. M., & El-Kadi, A. O. (2008). The role of redox-sensitive transcription factors NF-kappaB and AP-1 in the modulation of the Cyp1a1 gene by mercury, lead, and copper. Free Radical Biology and Medicine, 44, 795–806.
Maayah, Z. H., Ansari, M. A., El Gendy, M. A., Al-Arifi, M. N., & Korashy, H. M. (2014). Development of cardiac hypertrophy by sunitinib in vivo and in vitro rat cardiomyocytes is influenced by the aryl hydrocarbon receptor signaling pathway. Archives of Toxicology, 88, 725–738.
Clark, J. E., Sarafraz, N., & Marber, M. S. (2007). Potential of p38-MAPK inhibitors in the treatment of ischaemic heart disease. Pharmacology and Therapeutics, 116, 192–206.
Zhao, Y., Xue, T., Yang, X., Zhu, H., Ding, X., Lou, L., et al. (2010). Autophagy plays an important role in sunitinib-mediated cell death in H9c2 cardiac muscle cells. Toxicology and Applied Pharmacology, 248, 20–27.
Kimes, B. W., & Brandt, B. L. (1976). Properties of a clonal muscle cell line from rat heart. Experimental Cell Research, 98, 367–381.
Watkins, S. J., Borthwick, G. M., & Arthur, H. M. (2011). The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. In Vitro Cellular and Developmental Biology Animal, 47, 125–131.
Chen, Q. M., Tu, V. C., Wu, Y., & Bahl, J. J. (2000). Hydrogen peroxide dose dependent induction of cell death or hypertrophy in cardiomyocytes. Archives of Biochemistry and Biophysics, 373, 242–248.
Zordoky, B. N., Aboutabl, M. E., & El-Kadi, A. O. (2008). Modulation of cytochrome P450 gene expression and arachidonic acid metabolism during isoproterenol-induced cardiac hypertrophy in rats. Drug Metabolism and Disposition, 36, 2277–2286.
French, K. J., Coatney, R. W., Renninger, J. P., Hu, C. X., Gales, T. L., Zhao, S., et al. (2010). Differences in effects on myocardium and mitochondria by angiogenic inhibitors suggest separate mechanisms of cardiotoxicity. Toxicologic Pathology, 38, 691–702.
Reiser, P. J., Portman, M. A., Ning, X. H., & Schomisch Moravec, C. (2001). Human cardiac myosin heavy chain isoforms in fetal and failing adult atria and ventricles. American Journal of Physiology Heart and Circulatory Physiology, 280, H1814–H1820.
Hydock, D. S., Wonders, K. Y., Schneider, C. M., & Hayward, R. (2009). Voluntary wheel running in rats receiving doxorubicin: effects on running activity and cardiac myosin heavy chain. Anticancer Research, 29, 4401–4407.
Lee, H. S., Son, C. B., Shin, S. H., & Kim, Y. S. (2008). Clinical correlation between brain natriutetic peptide and anthracyclin-induced cardiac toxicity. Cancer Research and Treatment, 40, 121–126.
Jarolim, P. (2006). Serum biomarkers for heart failure. Cardiovascular Pathology, 15, 144–149.
Spallarossa, P., Altieri, P., Aloi, C., Garibaldi, S., Barisione, C., Ghigliotti, G., et al. (2009). Doxorubicin induces senescence or apoptosis in rat neonatal cardiomyocytes by regulating the expression levels of the telomere binding factors 1 and 2. American Journal of Physiology Heart and Circulatory Physiology, 297, H2169–H2181.
Guo, R. M., Xu, W. M., Lin, J. C., Mo, L. Q., Hua, X. X., Chen, P. X., et al. (2013). Activation of the p38 MAPK/NF-kappaB pathway contributes to doxorubicin-induced inflammation and cytotoxicity in H9c2 cardiac cells. Molecular Medicine Reports, 8, 603–608.
Huang, X. Z., Li, Z. R., Zhu, L. B., Huang, H. Y., Hou, L. L., & Lin, J. (2014). Inhibition of p38 mitogen-activated protein kinase attenuates butyrate-induced intestinal barrier impairment in A Caco-2 cell monolayer model. Journal of pediatric Gastroenterology and Nutrition (in press).
Chen, R., Li, X., Lu, S., Ma, T., Huang, X., Mylonakis, E., Liang, Y., & Xi, L. (2014). Role of extracellular signal-regulated kinases 1 and 2 and p38 mitogen-activated protein kinase pathways in regulating replication of Penicillium marneffei in human macrophages. Microbes and Infection, 16, 401–408.
Choi, H., Nguyen, H.N., & Lamb, F.S. (2014). Inhibition of endocytosis exacerbates TNFalpha-induced endothelial dysfunction via enhanced JNK and p38 activation. American Journal of Physiology Heart Circulatory Physiology, 306, H1154–H1163.
Park, G. B., Choi, Y., Kim, Y. S., Lee, H. K., Kim, D., & Hur, D. Y. (2014). ROS-mediated JNK/p38-MAPK activation regulates Bax translocation in Sorafenib-induced apoptosis of EBV-transformed B cells. International Journal of Oncology, 44, 977–985.
Su, X., Wang, X., Zhang, K., Yang, S., Xue, Q., Wang, P., & Liu, Q. (2014). ERK inhibitor U0126 enhanced SDT-induced cytotoxicity of human leukemia U937 cells. General Physiology Biophysics (in press).
Randhawa, H., Kibble, K., Zeng, H., Moyer, M. P., & Reindl, K. M. (2013). Activation of ERK signaling and induction of colon cancer cell death by piperlongumine. Toxicology In Vitro, 27, 1626–1633.
Qin, W., Liu, P., Zhang, R., Huang, S., Gao, X., Song, Z., Wang, R., Chen, L., Guo, B., & Lin, Z. (2014). JNK MAPK is involved in BMP-2-induced odontoblastic differentiation of human dental pulp cells. Connective Tissue Research, 55, 217–224.
Acknowledgments
This work is supported by the King Abdulaziz City for Science and Technology (KACST) Grant # A-S-10-0192 and the College of Pharmacy Research Center, King Saud University, Saudi Arabia.
Conflict of interest
There are no financial or other interests with regard to this manuscript that might be construed as a conflict of interest. All of the authors are aware of and agree to the content of the manuscript and their being listed as an author on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Korashy, H.M., Al-Suwayeh, H.A., Maayah, Z.H. et al. Mitogen-Activated Protein Kinases Pathways Mediate the Sunitinib-Induced Hypertrophy in Rat Cardiomyocyte H9c2 Cells. Cardiovasc Toxicol 15, 41–51 (2015). https://doi.org/10.1007/s12012-014-9266-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-014-9266-y