Abstract
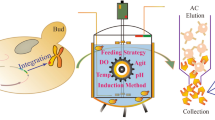
To confirm the treating effectiveness of midkine as an articular protective agent, mouse midkine (mMK) was produced for the pre-clinic long-term studies in mice. The protein was expressed under the control of the AOX1 gene promoter in Pichia pastoris, X-33 strain, and secreted into fermentation broth through high-density fermentation. Approximately 380 mg mMK, containing authentic and truncated forms, was secreted into 1 liter induction medium and 280 mg mMK was obtained after one-step purification on a 50 ml SP Sepharose Fast Flow column. The purified protein was characterized and identified to be the mature, authentic form of mMK. N-terminal five amino acid sequence was determined to be K-K-K-E-K. SDS-PAGE analysis indicated that the molecular weight of the product was about 13 KDa. The purity of the purified rmMK protein was determined to be 99 % by high performance liquid chromatography. The biological activity of final product was verified via migration assay on osteoblast-like UMR-106 cells.





Similar content being viewed by others
References
Kadomatsu, K., Tomomura, M., & Muramatsu, T. (1988). cDNA cloning and sequencing of a new gene intensely expressed in early differentiation stages of embryonal carcinoma cells and in mid-gestation period of mouse embryogenesis. Biochemical and Biophysical Research Communications, 151, 1312–1318.
Milner, P. G., Li, Y. S., Hoffman, R. M., Kodner, C. M., Siegel, N. R., & Deuel, T. F. (1989). A novel 17 kD heparin-binding growth factor (HBGF-8) in bovine uterus: purification and N-terminal amino acid sequence. Biochemical and Biophysical Research Communications, 165, 1096–1103.
Fabri, L., Maruta, H., Muramatsu, H., Muramatsu, T., Simpson, R. J., Burgess, A. W., et al. (1993). Structural characterisation of native and recombinant forms of the neurotrophic cytokine MK. Journal of Chromatography, 646, 213–225.
Akhter, S., Ichihara-Tanaka, K., Kojima, S., Muramatsu, H., Inui, T., Kimura, T., et al. (1998). Clusters of basic amino acids in midkine: roles in neurite-promoting activity and plasminogen activator-enhancing activity. Journal of Biochemistry, 123, 1127–1136.
Kojima, S., Inui, T., Muramatsu, H., Suzuki, Y., Kadomatsu, K., Yoshizawa, M., et al. (1997). Dimerization of midkine by tissue transglutaminase and its functional implication. Journal of Biological Chemistry, 272, 9410–9416.
Muramatsu, T. (2014). Structure and function of midkine as the basis of its pharmacological effects. British Journal of Pharmacology, 171, 814–826.
Tsutsui, J., Kadomatsu, K., Matsubara, S., Nakagawara, A., Hamanoue, M., Takao, S., et al. (1993). A new family of heparin-binding growth/differentiation factors: increased midkine expression in Wilms’ tumor and other human carcinomas. Cancer Research, 53, 1281–1285.
Aridome, K., Tsutsui, J., Takao, S., Kadomatsu, K., Ozawa, M., Aikou, T., et al. (1995). Increased midkine gene expression in human gastrointestinal cancers. Japanese Journal of Cancer Research, 86, 655–661.
Kadomatsu, K., & Muramatsu, T. (2004). Midkine and pleiotrophin in neural development and cancer. Cancer Letters, 204, 127–143.
Muramatsu, T. (2010). Midkine, a heparin-binding cytokine with multiple roles in development, repair and diseases. Proceedings of the Japan Academy. Series B, Physical and Biological Sciences, 86, 410–425.
Muramatsu, T. (2011). Midkine: a promising molecule for drug development to treat diseases of the central nervous system. Current Pharmaceutical Design, 17, 410–423.
Zhang, Z. H., Li, H. X., Qi, Y. P., Du, L. J., Zhu, S. Y., Wu, M. Y., et al. (2010). Recombinant human midkine stimulates proliferation of articular chondrocytes. Cell Proliferation, 43, 184–194.
Tsutsui, J., Uehara, K., Kadomatsu, K., Matsubara, S., & Muramatsu, T. (1991). A new family of heparin-binding factors: strong conservation of midkine (MK) sequences between the human and the mouse. Biochemical and Biophysical Research Communications, 176, 792–797.
Inui, T., Bodi, J., Kubo, S., Nishio, H., Kimura, T., Kojima, S., et al. (1996). Solution synthesis of human midkine, a novel heparin-binding neurotrophic factor consisting of 121 amino acid residues with five disulphide bonds. Journal of Peptide Science, 2, 28–39.
Zhang, Z. H., Du, L. J., Xiang, D., Zhu, S. Y., Wu, M. Y., Lu, H. L., et al. (2009). Expression and purification of bioactive high-purity human midkine in Escherichia coli. Journal of Zhejiang University. Science. B, 10, 79–86.
Murasugi, A., & Tohma-Aiba, Y. (2003). Production of native recombinant human midkine in the yeast, Pichia pastoris. Protein Expression and Purification, 27, 244–252.
Murasugi, A., & Tohma-Aiba, Y. (2002). Characterization of partially truncated human midkine expressed in Pichia pastoris. Bioscience Biotechnology and Biochemistry, 66, 1295–1300.
Qian, L., Zhu, S., Shen, J., Han, X., Gao, J., Wu, M., et al. (2012). Expression and purification of recombinant human Mig in Escherichia coli and its comparison with murine Mig. Protein Expression and Purification, 82, 205–211.
Xiang, D., Zhang, J., Chen, Y., Guo, Y., Schalow, A., Zhang, Z., et al. (2010). Expressions and purification of a mature form of recombinant human Chemerin in Escherichia coli. Protein Expression and Purification, 69, 153–158.
Matsuda, Y., Talukder, A. H., Ishihara, M., Hara, S., Yoshida, K., Muramatsu, T., et al. (1996). Limited proteolysis by chymotrypsin of midkine and inhibition by heparin binding. Biochemical and Biophysical Research Communications, 228, 176–181.
Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N., & Sternberg, M. J. (2015). The Phyre2 web portal for protein modeling, prediction and analysis. Nature Protocols, 10, 845–858.
Pacher, P., Beckman, J. S., & Liaudet, L. (2007). Nitric oxide and peroxynitrite in health and disease. Physiological Reviews, 87, 315–424.
Maitra, U., Gan, L., Chang, S., & Li, L. (2011). Low-dose endotoxin induces inflammation by selectively removing nuclear receptors and activating CCAAT/enhancer-binding protein delta. Journal of Immunology, 186, 4467–4473.
Akira, S. (2001). Toll-like receptors and innate immunity. Advances in Immunology, 78, 1–56.
Malyala, P., & Singh, M. (2008). Endotoxin limits in formulations for preclinical research. Journal of Pharmaceutical Sciences, 97, 2041–2044.
Qi, M., Ikematsu, S., Maeda, N., Ichihara-Tanaka, K., Sakuma, S., Noda, M., et al. (2001). Haptotactic migration induced by midkine. Involvement of protein-tyrosine phosphatase zeta. Mitogen-activated protein kinase, and phosphatidylinositol 3-kinase. Journal of Biological Chemistry, 276, 15868–15875.
Narayanan, S. S., & Nampoothiri, K. M. (2012). Biochemical characterization of recombinant methionine aminopeptidases (MAPs) from Mycobacterium tuberculosis H37Rv. Molecular and Cellular Biochemistry, 365, 191–202.
Acknowledgements
Financial support for this work was provided by National Science Foundation of China (Grant No. 81173113 and Grant No. 81273573), Shanghai Science Foundation (Grant No. 11431921300) and National Post-doctor Foundation of China (Grant No. 2012M520902).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jin Gao and Haixia Wang contributed equally to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Figure S1
3D structure of rmMK. The structure was predicted with software Phyre2 and viewed with a Java viewer Jmol. (TIFF 154 kb)
Rights and permissions
About this article
Cite this article
Gao, J., Wang, H., Li, J. et al. Eukaryotic Expression and Purification of Native Form of Mouse Midkine from Pichia pastoris . Appl Biochem Biotechnol 178, 490–503 (2016). https://doi.org/10.1007/s12010-015-1889-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1889-3