Opinion statement
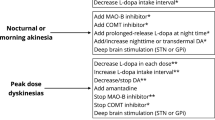
Motor fluctuations represent important late complications of Parkinson’s disease treated with levodopa. Although treatment of these problems has improved with the emergence of numerous pharmacologic and surgical therapies, the various options can make it confusing. Pharmacologic treatment is the first step. Polytherapy is often the rule in this case with a variety of agents available as adjunctive therapy with levodopa. These adjuncts include dopamine agonists (bromocriptine, pergolide, pramipexole, ropinirole), catechol-O-methyltransferase (COMT) inhibitors (tolcapone), controlled-release formulations of levodopa, monoamine oxidase (MAO) B inhibitors (selegiline), and amantadine. The treatment can consist of any of a number of combinations of these agents. No single algorithm can be used in all patients; therapy should be individualized. Physicians treating these patients need to be well versed in late complication patterns as well as the medications chosen. In addition, optimal doses vary, and often patients are considered treatment failures an taken off medications before reaching that level. In the more complicated cases, patients should be evaluated by specialists in movement disorders.
With this in mind, some guidelines are offered for the pharmacologic approach to patients with fluctuating responses to medications. For simple wearing off, controlledrelease levodopa (Sinemet CR, Dupont Pharmaceuticals, Wilmington, DE), COMT inhibitors MAO inhibitors, and dopamine agonists are reasonable options. For more complicated fluctuations, dopamine agonists with limits on levodopa are the first choice, especially when dyskinesia is present; when dyskinesia is not a factor, COMT inhibitors may be used. For dyskinesia specifically, dopamine agonists or addition of amantadine can be helpful.
Surgery should be a treatment of last resort for patients in whom medical therapy fails. Patients who are candidates for medial pallidotomy should be fluctuators with severe dyskinesia and “off” periods that have not improved with pharmacologic therapy. Thalami deep brain stimulation (DBS) should be used only in patients with tremor-predominant disease and severe intractable tremor that is unresponsive to medication and occurs not only at rest but with posture and action as well. Surgical therapy should be performed only in centers with surgeons experienced in stereotactic techniques and movement disorder specialists to ensure that the appropriate patients come to surgery and that complications are kept to a minimum.
Dietary adjustment has a limited role in treating advanced Parkinson’s disease.
Similar content being viewed by others
References and Recommended Reading
Weiner WJ, Lang AE: Movement Disorders: A Comprehensive Survey. Mount Kisco, NY: Futura; 1989.
Agid Y, Javoy-Agid F, Ruberg M: Biochemistry of neurotransmitters in Parkinson’s disease. In Movement Disorders, edn 2. Edited by Marsden CD, Fahn S. London: Butterworth; 1987:166–230.
Marsden CD, Parkes JD, Quinn N: Fluctuations of disability in Parkinson’s disease: clinical aspects. In Movement Disorders. Edited by Marsden CD, Fahn S. London: Butterworth Scientific; 1982:96–122.
Fabbrini G, Mouradian MM, Juncos JL, et al.: Motor fluctuations in Parkinson’s disease: central pathophysiological mechanisms, part I. Ann Neurol 1988, 24:366–371.
Bravi D, Mouradian MM, Roberts JW, et al.: Wearing-off fluctuations in Parkinson’s disease: contributions of postsynaptic mechanisms. Ann Neurol 1994, 36:27–31.
Mouradian MM, Juncos JL, Fabbrini G, et al.: Motor fluctuations in Parkinson’s disease: central pathophysiological mechanisms, part II. Ann Neurol 1988, 24:372–378.
Verhagan Metman L, Locatelli ER, Bravi D, et al.: Apomorphine responses in Parkinson’s disease and the pathogenesis of motor complications. Neurolog 1997, 48:369–372.
Djaldetti R, Baron J, Ziv I, Melamed E: Gastric emptying in Parkinson’s disease: patients with and without response fluctuations. Neurology 1996, 46:1051.
Nutt JG, Woodward WR, Carter JH, Trotman TL: Influence of fluctuations of plasma large neutral amino acids and normal diets on clinical response to levodopa. J Neurol Neurosurg Psychiatr 1989, 52:481–487.
Mouradian MM, Heuser JE, Baronti F, Chase TN: Modification of central dopaminergic mechanisms by continuous levodopa therapy for advanced Parkinson’s disease. Ann Neurol 1990, 27:18–23.
Riley D, Lang AE: Practical application of a low-protein diet for Parkinson’s disease. Neurology 1988, 38:1026–1031.
Berry EM, Growden JH, Wurtman JJ, et al.: A balanced carbohydrate: protein diet in the management of Parkinson’s disease. Neurolog 1991, 41:1295–1297.
Guttman M, International Pramipexole-Bromocriptine Study Group: Double-blind comparison of pramipexole and bromocriptine treatment with placebo in advanced Parkinson’s disease. Neurolog 1997, 49:1060–1065.
Olanow CW, Fahn S, Muenter M, et al.: A multicenter double-blind placebo-controlled trial of pergolide as an adjunct to Sinemet in Parkinson’s disease. Mov Disord 1994, 9:40–47.
Lieberman AN, Ranhosky A, Korts D: Clinical evaluation of pramipexole in advanced Parkinson’s disease: results of a double-blind, placebo-controlled, parallel-group study. Neurology 1997, 49:162–168.
Lieberman AN, Olanow CW, Sethi K, et al.: A multicenter trial of ropinirole as an adjunct treatment for Parkinson’s disease. Neurology 1998, 51:1057–1062. Ropinirole is the latest dopamine agonist approved for PD in the United States. This multicenter, randomized, double-blind, placebo-controlled study evaluated the efficacy and safety of ropinirole in 149 patients with fluctuating PD. It demonstrated a significant decrease in “off” time and levodopa dose.
Baas H, Beiske AG, Ghika J, et al.: Catechol O-methyltransferase inhibition with tolcapone reduces the “wearing off” phenomenon and levodopa requirements in fluctuating parkinsonian patients. J Neurol Neurosurg Psychiatr 1997, 63:421–428.
Rajput AH, Martin W, Saint-Hilaire M-H, et al.: Tolcapone improves motor function in parkinsonian patients with the “wearing-off” phenomenon: a double-blind, placebocontrolled, multicenter trial. Neurology 1997, 49:1066–1071.
Adler CH, Singer C, O’Brien C, et al.: Randomized, placebo-controlled study of tolcapone in patients with fluctuating Parkinson’s disease treated with levodopa-carbidopa. Arch Neurol 1998, 55:1089–1095. Tolcapone is the newest anti-PD medication to be approved in the United States. This is the most recent double-blind, placebo-controlled study comparing tolcapone (100 mg tid, 200 mg tid, and placebo) in 215 patients with PD with motor fluctuations and dyskinesia. It demonstrated a significant increase in “on” time (more than 2 h/d) and decrease in levodopa dose requirement.
Ahlskog JE, Muenter MD, McManis PG, et al.: Controlledrelease Sinemet (CR4): a double blind crossover study in patients with fluctuating Parkinson’s disease. Mayo Clin Proc 1988, 63:876–886.
Cedarbaum JM, Hoey M, McDowell F: A double-blind crossover comparison of Sinemet CR4 and standard Sinemet 25/100 in patients with Parkinson’s disease and fluctuating motor performance. J Neurol Neurosurg Psychiatr 1989, 52:207–212.
Factor SA, Sanchez-Ramos JR, Weiner WJ, Ingenito AM: Efficacy of Sinemet CR4 in subgroups of patients with Parkinson’s disease. J Neurol Neurosurg Psychiatr 1989, 52:83–88.
Pahwa R, Busenbark K, Huber SJ, et al.: Clinical experience with controlled-release carbidopa/levodopa in Parkinson’s disease. Neurolog 1993, 43:677–681.
Stoof JC, Booij J, Durkarch B, Wolters EC: The anti-parkinsonian drug amantadine inhibits the N-methyl-D-aspartic acid-evoked release of acetylcholine from rat striatum in a noncompetitive way. Eur J Pharmacol 1992, 213:439–443.
Uitti RJ, Rajput AH, Ahlskog JE, et al.: Amantadine treatment is an independent predictor of improved survival in Parkinson’s disease. Neurolog 1996, 46:1551–1556.
Verhagen Metman L, Del Dotto P, van den Muncklof P, et al.: Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson’s disease. Neurology 1998, 50:1323–1326.
Rajput AH, Uitti RJ, Lang AE, et al.: Amantadine (Amd) ameliorates levodopa (LD) induced dyskinesia (DK). Neurology 1997, 48:A328.
Golbe LI, Lieberman AN, Muenter MD, et al.: Deprenyl in the treatment of symptom fluctuations in advanced Parkinson’s disease. Clin Neuropharmacol 1988, 11:45–55.
Golbe LI: Deprenyl as symptomatic therapy in Parkinson’s disease. Clin Neuropharmacol 1988, 11:387–400.
Lang AE, Lozano AM, Montgomery E, et al.: Posteroventral medial pallidotomy in advanced Parkinson’s disease. N Engl J Med 1997, 337:1036–1042. This paper reports results of pallidotomy in the largest cohort of patients with advanced PD. The most significant finding was a more than 80% decrease in contralateral dyskinesias.
Kishore A, Turnbull IM, Snow BJ, et al.: Efficacy, stability and predictors of outcome of pallidotomy for Parkinson’s disease: six-month follow-up with additional 1-year observations. Brain 1997, 120:729–737.
Shannon KM, Penn RD, Kroin JS, et al.: Stereotactic pallidotomy for the treatment of Parkinson’s disease: efficacy and adverse effects at 6 months in 26 patients. Neurology 1998, 50:434–438.
Samuel M, Caputo E, Brooks DJ, et al.: A study of medial pallidotomy for Parkinson’s disease: clinical outcome, MR location and complications. Brain 1998, 121:59–75.
Koller W, Pahwa R, Busenbark K, et al.: High frequenc unilateral thalamic stimulation in the treatment of essential and parkinsonian tremor. Ann Neurol 1997, 42:292–299.
Ondo W, Jankovic J, Schwartz K, et al.: Unilateral thalamic deep brain stimulation for refractory essential tremor and Parkinson’s disease tremor. Neurology 1998, 51:1063–1069.
Parkinson Study Group: Entacapone improves motor fluctuations in levodopa-treated Parkinson’s disease patients. Ann Neurol 1998, 42:747–755.
Marek K, Friedman J, Hauser R, et al.: Phase II evaluation of rasagiline mesylate (TVP-1012), a novel anti-parkinsonian drug in parkinsonian patients not using levodopa/carbidopa. Mov Disord 1997, 12:838–839.
Limousin P, Krack P, Pollack P, et al.: Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 1998, 339:1105–1111.
Pahwa R, Williamson S, Smith D, et al.: High frequenc stimulation of the globus pallidus for the treatment of Parkinson’s disease. Neurology 1997, 49:249–253.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Factor, S.A. Parkinson’s disease: Motor fluctuations. Curr Treat Options Neurol 1, 21–32 (1999). https://doi.org/10.1007/s11940-999-0029-1
Issue Date:
DOI: https://doi.org/10.1007/s11940-999-0029-1