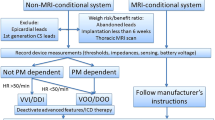
Opinion statement
The use of magnetic resonance imaging (MRI) as a diagnostic technique is rapidly expanding. The number of patients who undergo implantation of permanent pacemaker, implantable cardioverter defibrillator (ICD), and/or cardiac resynchronization therapy devices is increasing in parallel. Cardiovascular implants are subject to potentially harmful effects from MRI, and the routine use of this imaging modality for patients with standard cardiovascular devices is contraindicated. Several recent publications have suggested that MRI can safely be performed in standard cardiovascular device recipients with appropriate patient selection, device programming, and strict monitoring. In addition, MRI “conditional” device systems are now available that are specifically designed to be safe in the MRI environment. Such new technologies may simplify and improve overall safety of MRI in the setting of pacemaker and ICD systems. Although the availability of MRI conditional devices represents a significant breakthrough, their current use is limited to specific MRI conditions. MRI conditional cardiac device technology will likely continue to evolve with increased efforts to improve simplicity, safety, and generalizability under all MRI conditions.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Kalin R, Stanton MS. Current clinical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin Electrophysiol: PACE. 2005;28:326–8.
Levine GN, Gomes AS, Arai AE, et al. Safety of magnetic resonance imaging in patients with cardiovascular devices: an American Heart Association scientific statement from the Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Council on Cardiovascular Radiology and Intervention: endorsed by the American College of Cardiology Foundation, the North American Society for Cardiac Imaging, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2007;116:2878–91.
Roguin A, Zviman MM, Meininger GR, et al. Modern pacemaker and implantable cardioverter/defibrillator systems can be magnetic resonance imaging safe: in vitro and in vivo assessment of safety and function at 1.5 T. Circulation. 2004;110:475–82.
Nazarian S, Halperin HR. How to perform magnetic resonance imaging on patients with implantable cardiac arrhythmia devices. Heart Rhythm. 2009;6:138–43.
Tandri H, Zviman MM, Wedan SR, et al. Determinants of gradient field-induced current in a pacemaker lead system in a magnetic resonance imaging environment. Heart Rhythm. 2008;5:462–8.
Sommer T, Naehle CP, Yang A, et al. Strategy for safe performance of extrathoracic magnetic resonance imaging at 1.5 tesla in the presence of cardiac pacemakers in non-pacemaker-dependent patients: a prospective study with 115 examinations. Circulation. 2006;114:1285–92.
Vahlhaus C. Heating of pacemaker leads during magnetic resonance imaging. Eur Heart J. 2005;26:1243. author reply 1243–1244.
Nazarian S, Roguin A, Zviman MM, et al. Clinical utility and safety of a protocol for noncardiac and cardiac magnetic resonance imaging of patients with permanent pacemakers and implantable-cardioverter defibrillators at 1.5 tesla. Circulation. 2006;114:1277–84.
Roguin A, Schwitter J, Vahlhaus C, et al. Magnetic resonance imaging in individuals with cardiovascular implantable electronic devices. Europace : European pacing, arrhythmias, and cardiac electrophysiology : Journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology 2008;10:336–346.
Nazarian S, Hansford R, Roguin A, et al. A prospective evaluation of a protocol for magnetic resonance imaging of patients with implanted cardiac devices. Ann Intern Med. 2011;155:415–24.
American Society for Testing and Materials (ASTM) International, Designation: F2503-08, standard practice for marking medical devices and other items for safety in the magnetic resonance environment. ASTM International, West Conshohocken, PA.
United States Food and Drug Administration February 2011 PMA Approvals. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/PMAApprovals/ucm250089.htm?utm_campaign=Google2&utm_source=fdaSearch&utm_medium=website&utm_term=revo&utm_content=4. Accessed April 2012.
St. Jude Medical - Products. http://www.sjmprofessional.com/Products/index.aspx. Accessed April 2012.
Biotronik - Product manuals and warranties. http://www.biotronikusa.com/manuals/. Accessed April 2012.
Wilkoff BL, Bello D, Taborsky M, et al. Magnetic resonance imaging in patients with a pacemaker system designed for the magnetic resonance environment. Heart Rhythm. 2011;8:65–73.
Forleo GB, Santini L, Della Rocca DG, et al. Safety and efficacy of a new magnetic resonance imaging-compatible pacing system: early results of a prospective comparison with conventional dual-chamber implant outcomes. Heart Rhythm. 2010;7:750–4.
Medtronic - Revo MRI® SureScan® Pacing System Description. http://www.medtronic.com/mrisurescan-us/pacing_system_description.html. Accessed April 2012.
Gimbel JR. Magnetic resonance imaging of implantable cardiac rhythm devices at 3.0 tesla. Pacing Clin Electrophysiol: PACE. 2008;31:795–801.
Gimbel JR. Unexpected asystole during 3T magnetic resonance imaging of a pacemaker-dependent patient with a ‘modern’ pacemaker. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology 2009;11:1241–1242.
Gimbel JR. Unexpected pacing inhibition upon exposure to the 3 T static magnetic field prior to imaging acquisition: what is the mechanism? Heart Rhythm. 2011;8:944–5.
Disclosures
R. Beinart: none; S. Nazarian: Received honoraria for lectures (not speaker’s bureau) from SJM, MDT, Biotronic, and BSC and is on the MRI advisory panel (unpaid) for Medtronic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beinart, R., Nazarian, S. MRI-Conditional Cardiac Implantable Electronic Devices: What’s New and What Can We Expect in the Future?. Curr Treat Options Cardio Med 14, 558–564 (2012). https://doi.org/10.1007/s11936-012-0197-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11936-012-0197-2