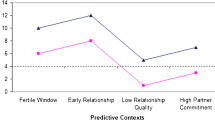
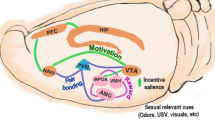
Abstract
Purpose of Review
The aim of this review is to provide current evidence on the biological and psychological mechanisms that underlie sexual partner preferences (SPP) in humans and animals.
Recent Findings
SPP depend mainly on prenatal (adaptive) organization of the brain, but can be drastically modified via learning under enhanced dopaminergic (DA) and oxytocinergic (OT) activity.
Summary
SPP can be categorized as in those directed towards partners who display indicators of biological fitness (IBF) or towards partners who do not show those indicators. The IBF function as unconditioned stimuli that presumably activate prenatally organized brain areas that mediate the salience of those stimuli. However, we discuss some evidence indicating that SPP not directed towards IBF (i.e., paraphilias) might be consequence of a learning process that occurs under enhanced DA or OT activity, resulting in new powerful learning with additional brain areas involved.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Seto MC. The puzzle of male chronophilias. Arch Sex Behav. 2017;46(1):3–22. https://doi.org/10.1007/s10508-016-0799-y. This study suggests that chronophilias might represent sexual orientations.
•• Bailey JM, Hsu KJ. Orienting basic research on chronophilias. Arch Sex Behav. 2017;46(1):23–6. https://doi.org/10.1007/s10508-016-0885-1. This study suggest that paraphilias might represent failure in attractions towards fertile partners.
Pfaus JG, Frank A. Beach award. Homologies of animal and human sexual behaviors. Horm Behav. 1996;30(3):187–200. https://doi.org/10.1006/hbeh.1996.0024.
Safron A, Barch B, Bailey JM, Gitelman DR, Parrish TB, Reber PJ. Neural correlates of sexual arousal in homosexual and heterosexual men. Behav Neurosci. 2007;121(2):237–48. https://doi.org/10.1037/0735-7044.121.2.237.
Cerny JA, Janssen E. Patterns of sexual arousal in homosexual, bisexual, and heterosexual men. Arch Sex Behav. 2011;40(4):687–97. https://doi.org/10.1007/s10508-011-9746-0.
Pfaus JG, Kippin TE, Coria-Avila GA, Gelez H, Afonso VM, Ismail N, et al. Who, what, where, when (and maybe even why)? How the experience of sexual reward connects sexual desire, preference, and performance. Arch Sex Behav. 2012;41(1):31–62. https://doi.org/10.1007/s10508-012-9935-5.
Damsma G, Pfaus JG, Wenkstern D, Phillips AG, Fibiger HC. Sexual behavior increases dopamine transmission in the nucleus accumbens and striatum of male rats: comparison with novelty and locomotion. Behav Neurosci. 1992;106(1):181–91.
Pfaus JG. Pathways of sexual desire. J Sex Med. 2009;6(6):1506–33. https://doi.org/10.1111/j.1743-6109.2009.01309.x.
Eleftheriou A, Bullock S, Graham CA, Stone N, Ingham R. Does attractiveness influence condom use intentions in heterosexual men? An experimental study. BMJ Open. 2016;6(6):e010883. https://doi.org/10.1136/bmjopen-2015-010883.
Kenrick DT, Sadalla EK, Groth G, Trost MR. Evolution, traits, and the stages of human courtship: qualifying the parental investment model. J Pers. 1990;58(1):97–116.
Rolls ET. Sexual behaviour, reward, and brain function; sexual selection of behaviour. In: Rolls ET, editor. Emotion explained. Great Britain: Oxford University Press; 2005. p. 358–99.
Dixson BJ, Dixson AF, Morgan B, Anderson MJ. Human physique and sexual attractiveness: sexual preferences of men and women in Bakossiland, Cameroon. Arch Sex Behav. 2007;36(3):369–75. https://doi.org/10.1007/s10508-006-9093-8.
Buss DM. Sex differences in human mate preferences: evolutionary hypotheses tested in 37 cultures. Behav Brain Sci. 1989;12:1–49.
Penton-Voak IS, Perrett DI, Castles DL, Kobayashi T, Burt DM, Murray LK, et al. Menstrual cycle alters face preference. Nature. 1999;399(6738):741–2. https://doi.org/10.1038/21557.
Debat V. Symmetry is beauty—or is it? The rise and fall of fluctuating asymmetry. Med Sci (Paris). 2016;32(8–9):774–80. https://doi.org/10.1051/medsci/20163208028.
Zaromatidis K, Carlo R, Racanello D. Sex, perceptions of attractiveness, and sensation seeking and ratings of the likelihood of having sexually transmitted diseases. Psychol Rep. 2004;94(2):633–6. https://doi.org/10.2466/pr0.94.2.633-636.
Hodges-Simeon CR, Gurven M, Puts DA, Gaulin SJ. Vocal fundamental and formant frequencies are honest signals of threat potential in peripubertal males. Behav Ecol. 2014;25(4):984–8. https://doi.org/10.1093/beheco/aru081.
Langlois JH, Kalakanis L, Rubenstein AJ, Larson A, Hallam M, Smoot M. Maxims or myths of beauty? A meta-analytic and theoretical review. Psychol Bull. 2000;126(3):390–423.
Singh D. Body shape and women’s attractiveness: the critical role of waist-to-hip ratio. Hum Nat. 1993;4(3):297–321. https://doi.org/10.1007/BF02692203.
Roberts SC, Havlicek J, Flegr J, Hruskova M, Little AC, Jones BC, et al. Female facial attractiveness increases during the fertile phase of the menstrual cycle. Proc Biol Sci. 2004;271(Suppl 5):S270–2. https://doi.org/10.1098/rsbl.2004.0174.
Gildersleeve KA, Haselton MG, Larson CM, Pillsworth EG. Body odor attractiveness as a cue of impending ovulation in women: evidence from a study using hormone-confirmed ovulation. Horm Behav. 2012;61(2):157–66. https://doi.org/10.1016/j.yhbeh.2011.11.005.
Carrito ML, Santos IM, Alho L, Ferreira J, Soares SC, Bem-Haja P, et al. Do masculine men smell better? An association between skin color masculinity and female preferences for body odor. Chem Senses. 2017;42(3):269–75. https://doi.org/10.1093/chemse/bjx004.
•• Ramirez-Rodriguez R, Perusquia-Cabrera D, Díaz-Estrada VX, Herrera-Covarrubias D, Carrillo P, García L, et al. Conditioned sexual arousal towards infants in adult male rats: A model of learned pedophilia? In: Neuroscience Sf, editor. Annual Meeting of the Society For Neuroscience. Washington, DC: Society For Neuroscience; 2017. This study indicates that pedophilia can be developed after a conditioning process, specially if condingency occurs under the effects of D2-type enhanced activity.
Pandita-Gunawardena R. Paraphilic infantilism. A rare case of fetishistic behaviour. Br J Psychiatry. 1990;157:767–70.
•• Cibrian-Llanderal T, Rosas-Aguilar V, Triana-Del Rio R, Perez CA, Manzo J, Garcia LI, et al. Enhaced D2-type receptor activity facilitates the development of conditioned same-sex partner preference in male rats. Pharmacol Biochem Behav. 2012;102(2):177–83. https://doi.org/10.1016/j.pbb.2012.04.007. This study shows that same-sex preference can be learned after a process of conditioning, specially under the effects of D2-type activity.
•• Triana-Del Rio R, Tecamachaltzi-Silvaran MB, Diaz-Estrada VX, Herrera-Covarrubias D, Corona-Morales AA, Pfaus JG, et al. Conditioned same-sex partner preference in male rats is facilitated by oxytocin and dopamine: effect on sexually dimorphic brain nuclei. Behav Brain Res. 2015;283:69–77. https://doi.org/10.1016/j.bbr.2015.01.019. This study shows the effects of dopamine D2-type and oxitocin in the development of conditioned same-sex partner preference.
Kaul A, Duffy S. Gerontophilia—a case report. Med Sci Law. 1991;31(2):110–4. https://doi.org/10.1177/002580249103100204.
Hamby S, Finkelhor D, Turner H. Perpetrator and victim gender patterns for 21 forms of youth victimization in the National Survey of Children’s Exposure to Violence. Violence Vict. 2013;28(6):915–39.
Keaney TC. “Man-some”: a review of male facial aging and beauty. J Drugs Dermatol. 2017;16(6):91–3.
Thornhill R, Gangestad SW. Facial attractiveness. Trends Cogn Sci. 1999;3(12):452–60.
Kerr KL, Rosero SJ, Doty RL. Odors and the perception of hygiene. Percept Mot Skills. 2005;100(1):135–41. https://doi.org/10.2466/pms.100.1.135-141.
Ferdenzi C, Schaal B, Roberts SC. Human axillary odor: are there side-related perceptual differences? Chem Senses. 2009;34(7):565–71. https://doi.org/10.1093/chemse/bjp037.
Wedekind C, Seebeck T, Bettens F, Paepke AJ. MHC-dependent mate preferences in humans. Proc Biol Sci. 1995;260(1359):245–9. https://doi.org/10.1098/rspb.1995.0087.
Heidekrueger PI, Szpalski C, Weichman K, Juran S, Ng R, Claussen C, et al. Lip attractiveness: a cross-cultural analysis. Aesthet Surg J. 2017;37(7):828–36. https://doi.org/10.1093/asj/sjw168.
• Coria-Avila GA, Manzo J, Garcia LI, Carrillo P, Miquel M, Pfaus JG. Neurobiology of social attachments. Neurosci Biobehav Rev. 2014;43:173–82. https://doi.org/10.1016/j.neubiorev.2014.04.004. This study summarizes the neurobiology of sexual partner preferences.
Pfaus JG, Damsma G, Nomikos GG, Wenkstern DG, Blaha CD, Phillips AG, et al. Sexual behavior enhances central dopamine transmission in the male rat. Brain Res. 1990;530(2):345–8.
Gingrich B, Liu Y, Cascio C, Wang Z, Insel TR. Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster). Behav Neurosci. 2000;114(1):173–83.
Hull EM, Du J, Lorrain DS, Matuszewich L. Testosterone, preoptic dopamine, and copulation in male rats. Brain Res Bull. 1997;44(4):327–33.
Newman R, Winans SS. An experimental study of the ventral striatum of the golden hamster. I. Neuronal connections of the nucleus accumbens. J Comp Neurol. 1980;191(2):167–92. https://doi.org/10.1002/cne.901910203.
Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978;180(3):545–80. https://doi.org/10.1002/cne.901800310.
Bartels A, Zeki S. The neural basis of romantic love. Neuroreport. 2000;11(17):3829–34.
Cavanaugh BL, Lonstein JS. Social novelty increases tyrosine hydroxylase immunoreactivity in the extended olfactory amygdala of female prairie voles. Physiol Behav. 2010;100(4):381–6. https://doi.org/10.1016/j.physbeh.2010.03.020.
Meloy JR, Fisher H. Some thoughts on the neurobiology of stalking. J Forensic Sci. 2005;50(6):1472–80.
Fiorino DF, Coury A, Phillips AG. Dynamic changes in nucleus accumbens dopamine efflux during the Coolidge effect in male rats. J Neurosci. 1997;17(12):4849–55.
Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40(2):133–8. https://doi.org/10.1006/hbeh.2001.1691.
Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21(20):8278–85.
Millan EZ, Kim HA, Janak PH. Optogenetic activation of amygdala projections to nucleus accumbens can arrest conditioned and unconditioned alcohol consummatory behavior. Neuroscience. 2017;360:106–17. https://doi.org/10.1016/j.neuroscience.2017.07.044.
Kruger TH, Haake P, Chereath D, Knapp W, Janssen OE, Exton MS, et al. Specificity of the neuroendocrine response to orgasm during sexual arousal in men. J Endocrinol. 2003;177(1):57–64.
Shipley MT, Ennis M. Functional organization of olfactory system. J Neurobiol. 1996;30(1):123–76. https://doi.org/10.1002/(SICI)1097-4695(199605)30:1<123::AID-NEU11>3.0.CO;2-N.
Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25(9):1565–74. https://doi.org/10.1016/j.peptides.2004.05.019.
Dhungel S, Masaoka M, Rai D, Kondo Y, Sakuma Y. Both olfactory epithelial and vomeronasal inputs are essential for activation of the medial amygdala and preoptic neurons of male rats. Neuroscience. 2011;199:225–34. https://doi.org/10.1016/j.neuroscience.2011.09.051.
Baum MJ, Everitt BJ. Increased expression of c-fos in the medial preoptic area after mating in male rats: role of afferent inputs from the medial amygdala and midbrain central tegmental field. Neuroscience. 1992;50(3):627–46.
Kelliher KR, Liu YC, Baum MJ, Sachs BD. Neuronal Fos activation in olfactory bulb and forebrain of male rats having erections in the presence of inaccessible estrous females. Neuroscience. 1999;92(3):1025–33.
Erskine MS, Hanrahan SB. Effects of paced mating on c-fos gene expression in the female rat brain. J Neuroendocrinol. 1997;9(12):903–12.
Coopersmith C, Gans SE, Rowe DW, Erskine MS. Infusions of lidocaine into the amygdala, but not the preoptic area, block pseudopregnancy in the rat. J Neuroendocrinol. 1996;8(4):259–66.
Gorski RA. Sexual differentiation of the brain. Hosp Pract. 1978;13(10):55–62.
Roselli CE, Reddy RC, Kaufman KR. The development of male-oriented behavior in rams. Front Neuroendocrinol. 2011;32(2):164–9. https://doi.org/10.1016/j.yfrne.2010.12.007.
Coria-Avila GA, Herrera-Covarrubias D, Paredes-Ramos P, Alvarez-Croda DM, Tecamachaltzi-Silvaran M, Rosales-Raya JB, et al. Dimorfismo cerebral y preferencia sexual en una rata pseudohermafrodita. e-Neurobiologia. 2014;5(9):090614.
Davis EC, Shryne JE, Gorski RA. A revised critical period for the sexual differentiation of the sexually dimorphic nucleus of the preoptic area in the rat. Neuroendocrinology. 1995;62(6):579–85.
Perakis A, Stylianopoulou F. Effects of a prenatal androgen peak on rat brain sexual differentiation. J Endocrinol. 1986;108(2):281–5.
Goto K, Koizumi K, Ohta Y, Hashi M, Fujii Y, Ohbo N, et al. Evaluation of general behavior, memory, learning performance, and brain sexual differentiation in F1 offspring males of rats treated with flutamide during late gestation. J Toxicol Sci. 2005;30(3):249–59.
Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;148(2):333–46.
Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–82.
Lephart ED, Call SB, Rhees RW, Jacobson NA, Weber KS, Bledsoe J, et al. Neuroendocrine regulation of sexually dimorphic brain structure and associated sexual behavior in male rats is genetically controlled. Biol Reprod. 2001;64(2):571–8.
Woodson JC, Balleine BW, Gorski RA. Sexual experience interacts with steroid exposure to shape the partner preferences of rats. Horm Behav. 2002;42(2):148–57.
Gorski RA. Sexual differentiation of the nervous system. In: Kandel ER, Jessell TM, editors. Principles of neural science; 2000. p. 1131–48.
Matsumoto A, Arai Y. Effect of androgen on sexual differentiation of synaptic organization in the hypothalamic arcuate nucleus: an ontogenetic study. Neuroendocrinology. 1981;33(3):166–9.
LeVay S. A difference in hypothalamic structure between heterosexual and homosexual men. Science. 1991;253(5023):1034–7.
Swaab DF, Gooren LJ, Hofman MA. Brain research, gender and sexual orientation. J Homosex. 1995;28(3–4):283–301.
Allen LS, Gorski RA. Sexual dimorphism of the anterior commissure and massa intermedia of the human brain. J Comp Neurol. 1991;312(1):97–104. https://doi.org/10.1002/cne.903120108.
Savic I, Berglund H, Lindstrom P. Brain response to putative pheromones in homosexual men. Proc Natl Acad Sci U S A. 2005;102(20):7356–61. https://doi.org/10.1073/pnas.0407998102.
Paul T, Schiffer B, Zwarg T, Kruger TH, Karama S, Schedlowski M, et al. Brain response to visual sexual stimuli in heterosexual and homosexual males. Hum Brain Mapp. 2008;29(6):726–35. https://doi.org/10.1002/hbm.20435.
Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40(3):655–64.
Dressing H, Obergriesser T, Tost H, Kaumeier S, Ruf M, Braus DF. Homosexual pedophilia and functional networks—an fMRI case report and literature review. Fortschr Neurol Psychiatr. 2001;69(11):539–44. https://doi.org/10.1055/s-2001-18380.
Burns JM, Swerdlow RH. Right orbitofrontal tumor with pedophilia symptom and constructional apraxia sign. Arch Neurol. 2003;60(3):437–40.
Sartorius A, Ruf M, Kief C, Demirakca T, Bailer J, Ende G, et al. Abnormal amygdala activation profile in pedophilia. Eur Arch Psychiatry Clin Neurosci. 2008;258(5):271–7. https://doi.org/10.1007/s00406-008-0782-2.
• Schiffer B, Krueger T, Paul T, de Greiff A, Forsting M, Leygraf N, et al. Brain response to visual sexual stimuli in homosexual pedophiles. J Psychiatry Neurosci. 2008;33(1):23–33. This study shows the role of the cortex in pedophilia.
Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J Neurosci. 2003;23(8):3483–90.
Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, et al. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9(1):133–9. https://doi.org/10.1038/nn1613.
Wang Z, Aragona BJ. Neurochemical regulation of pair bonding in male prairie voles. Physiol Behav. 2004;83(2):319–28. https://doi.org/10.1016/j.physbeh.2004.08.024.
Wang Z, Yu G, Cascio C, Liu Y, Gingrich B, Insel TR. Dopamine D2 receptor-mediated regulation of partner preferences in female prairie voles (Microtus ochrogaster): a mechanism for pair bonding? Behav Neurosci. 1999;113(3):602–11.
•• Coria-Avila GA, Cibrian-Llanderal T, Diaz-Estrada VX, Garcia LI, Toledo-Cardenas R, Pfaus JG, et al. Brain activation associated to olfactory conditioned same-sex partner preference in male rats. Horm Behav. 2018; https://doi.org/10.1016/j.yhbeh.2018.02.005. This study shows the brain areas activated in male rats that developed a conditioned same-sex partner preference.
• Diaz-Estrada VX, Tecamachaltzi-Silvaran M, Barradas-Moctezuma M, Herrera-Covarrubias D, Ismail N, Coria-Avila GA. Conditioned same-sex partner preference and testosterone levels in male rats. e-Neurobiologia. 2015;7(14):12012016. This study shows that conditioned same-sex partner preference does not modify serum testosterone.
Coria-Avila GA, Pfaus JG. Neuronal activation by stimuli that predict sexual reward in female rats. Neuroscience. 2007;148(3):623–32. https://doi.org/10.1016/j.neuroscience.2007.05.052.
Pfaus JG, Tse TL, Werk CM, Chanda ML, Leblonde A, Harbour VL, et al. Enhanced synaptic responses in the piriform cortex associated with sexual stimulation in the male rat. Neuroscience. 2009;164(4):1422–30. https://doi.org/10.1016/j.neuroscience.2009.09.060.
Robarts DW, Baum MJ. Ventromedial hypothalamic nucleus lesions disrupt olfactory mate recognition and receptivity in female ferrets. Horm Behav. 2007;51(1):104–13. https://doi.org/10.1016/j.yhbeh.2006.08.009.
•• Ramírez-Rodríguez R, Perusquía-Cabrera D, Herrera-Covarrubias D, García L, Manzo J, Coria-Avila GA. Excitación sexual condicionada hacia prepúberes en ratas macho adultas: ¿Un modelo de pedofilia aprendida? In: Fisiológicas SMDC, editor. LX Congreso Nacional de la Sociedad Mexicana de Ciencias Fisiológicas. Monterrey: Sociedad Mexicana de Ciencias Fisiológicas; 2017. This study shows that pedophilia can be learned, specially under the effects of D2-type enhanced activity.
•• Hsu C. Parkinson’s medication turned respected family man into “gay sex and gambling addict”. Medical Daily. 2012. http://www.medicaldaily.com/parkinsons-medication-turned-respected-family-man-gay-sex-and-gambling-addict-243707. Accessed 28/04/2015. This case report indicates that dopaminergic medicine use for Parkinson’s disease can change sexual preference.
Acknowledgements
The authors want to thank CONACYT of Mexico for the grant support (GACA-167773 and S-8806). In addition, we thank the reviewers who helped us improve enormously the final version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Preclinical and Psychophysiology
Rights and permissions
About this article
Cite this article
Coria-Avila, G.A., Herrera-Covarrubias, D., Hernández, M.E. et al. Understanding Sexual Partner Preference: from Biological Diversity to Psychiatric Disorders. Curr Sex Health Rep 10, 142–151 (2018). https://doi.org/10.1007/s11930-018-0152-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11930-018-0152-7