Abstract
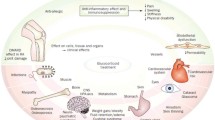
Glucocorticoids are often required for adequate control of inflammation in many serious inflammatory diseases; common indications for long-term treatment include polymyalgia rheumatica, giant cell arteritis, asthma and chronic obstructive pulmonary disease. Long-term glucocorticoid therapy is, however, associated with many adverse effects involving skin, gastro-intestinal, eye, skeletal muscle, bone, adrenal, cardio-metabolic and neuropsychiatric systems. This balance between benefits and risks of glucocorticoids is important for clinical practice and glucocorticoid-related adverse effects can significantly impair health-related quality of life. Understanding the nature and mechanisms of glucocorticoid-related adverse effects may inform how patients are monitored for toxicity and identify those groups, such as older people, that may need closer monitoring. For clinical trials in diseases commonly treated with glucocorticoids, standardised measurement of glucocorticoid-related adverse effects would facilitate future evidence synthesis and meta-analysis.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance;•• Of major importance
Hench P. Effects of cortisone in the rheumatic diseases. Lancet. 1950;2(6634):483–4.
Smolen, J.S., et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73(3):492–509.
Sarnes E et al. Incidence and US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Ther. 2011;33(10):1413–32.
Kirkham JJ et al. Can a core outcome set improve the quality of systematic reviews?—a survey of the Co-ordinating editors of Cochrane review groups. Trials. 2013;14:21.
Boers M et al. Developing core outcome measurement sets for clinical trials: OMERACT filter 2.0. J Clin Epidemiol. 2014;67(7):745–53.
Chen YF et al. Scientific hypotheses can be tested by comparing the effects of one treatment over many diseases in a systematic review. J Clin Epidemiol. 2014;67(12):1309–19.
Sheu HM et al. Depletion of stratum corneum intercellular lipid lamellae and barrier function abnormalities after long-term topical corticosteroids. Br J Dermatol. 1997;136(6):884–90.
Schoepe S et al. Glucocorticoid therapy-induced skin atrophy. Exp Dermatol. 2006;15(6):406–20.
Newell-Price J et al. Cushing’s syndrome. Lancet. 2006;367(9522):1605–17.
Schäcke H, Döcke W-D, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96(1):23–43.
Tiganescu A et al. 11β-Hydroxysteroid dehydrogenase blockade prevents age-induced skin structure and function defects. J Clin Invest. 2013;123(7):3051–60.
Kerscher MJ, Korting HC. Comparative atrophogenicity potential of medium and highly potent topical glucocorticoids in cream and ointment according to ultrasound analysis. Skin Pharmacol. 1992;5(2):77–80.
Curtis JR et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Care Res. 2006;55(3):420–6.
Vinding GR et al. Self-reported skin morbidities and health-related quality of life: a population-based nested case-control study. Dermatology. 2014;228(3):261–8.
Van der Goes MC et al. Monitoring adverse events of low-dose glucocorticoid therapy: EULAR recommendations for clinical trials and daily practice. Ann Rheum Dis. 2010;69(11):1913–9.
Storms WW, Theen C. Clinical adverse effects of inhaled corticosteroids: results of a questionnaire survey of asthma specialists. Ann Allergy Asthma Immunol. 1998;80(5):391–4.
Andrews RC et al. Abnormal cortisol metabolism and tissue sensitivity to cortisol in patients with glucose intolerance. J Clin Endocrinol Metab. 2002;87(12):5587–93.
Quondamatteo F. Skin and diabetes mellitus: what do we know? Cell Tissue Res. 2014;355(1):1–21.
Makrantonaki E, Zouboulis CC. Molecular mechanisms of skin aging: state of the art. Ann N Y Acad Sci. 2007;1119:40–50.
Aschoff R et al. Evaluation of the atrophogenic potential of hydrocortisone 1 % cream and pimecrolimus 1 % cream in uninvolved forehead skin of patients with atopic dermatitis using optical coherence tomography. Exp Dermatol. 2011;20(10):832–6.
Schoepe S, Schacke H, Asadullah K. Test systems for the determination of glucocorticoid receptor ligand induced skin atrophy. Dermatoendocrinol. 2011;3(3):175–9.
Norsgaard H et al. Calcipotriol counteracts betamethasone-induced decrease in extracellular matrix components related to skin atrophy. Arch Dermatol Res. 2014;306(8):719–29.
Hooten J, Hall 3rd R, Cardones A. Updates on the management of autoimmune blistering diseases. Skin Ther Lett. 2014;19(5):1–6.
Schacke H et al. Characterization of ZK 245186, a novel, selective glucocorticoid receptor agonist for the topical treatment of inflammatory skin diseases. Br J Pharmacol. 2009;158(4):1088–103.
Sen CK et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763–71.
Nagareddy PR et al. Increased expression of iNOS is associated with endothelial dysfunction and impaired pressor responsiveness in streptozotocin-induced diabetes. Am J Physiol Heart Circ Physiol. 2005;289(5):H2144–52.
Holmes CJ et al. Dynamic role of host stress responses in modulating the cutaneous microbiome: implications for wound healing and infection. Adv Wound Care (New Rochelle). 2015;4(1):24–37.
Da Silva JA et al. Safety of low dose glucocorticoid treatment in rheumatoid arthritis: published evidence and prospective trial data. Ann Rheum Dis. 2006;65(3):285–93. Epub 2005 Aug 17.
Gosain A, DiPietro LA. Aging and wound healing. World J Surg. 2004;28(3):321–6.
Brubaker AL et al. Reduced neutrophil chemotaxis and infiltration contributes to delayed resolution of cutaneous wound infection with advanced age. J Immunol. 2013;190(4):1746–57.
Saag KG et al. Low dose long-term corticosteroid therapy in rheumatoid arthritis: an analysis of serious adverse events. Am J Med. 1994;96(2):115–23.
van der Goes MC et al. Patient and rheumatologist perspectives on glucocorticoids: an exercise to improve the implementation of the European League Against Rheumatism (EULAR) recommendations on the management of systemic glucocorticoid therapy in rheumatic diseases. Ann Rheum Dis. 2010;69(6):1015–21.
Helliwell T, Hider SL, Mallen CD. Polymyalgia rheumatica: diagnosis, prescribing, and monitoring in general practice. Br J Gen Pract. 2013;63(610):e361–6.
Carli L et al. Analysis of the prevalence of cataracts and glaucoma in systemic lupus erythematosus and evaluation of the rheumatologists’ practice for the monitoring of glucocorticoid eye toxicity. Clin Rheumatol. 2013;32(7):1071–3.
Prokofyeva E, Wegener A, Zrenner E. Cataract prevalence and prevention in Europe: a literature review. Acta Ophthalmologica. 2013;91(5):395–405.
Rasanen P et al. Cost-utility of routine cataract surgery. Health Qual Life Outcomes. 2006;4:74.
Prokofyeva E, Zrenner E. Epidemiology of major eye diseases leading to blindness in Europe: a literature review. Ophthalmic Res. 2012;47(4):171–88.
Huscher D et al. Dose-related patterns of glucocorticoid-induced side effects. Ann Rheum Dis. 2009;68(7):1119–24.
Hoes JN et al. EULAR evidence-based recommendations on the management of systemic glucocorticoid therapy in rheumatic diseases. Ann Rheum Dis. 2007;66(12):1560–7.
Mosca M et al. European League Against Rheumatism recommendations for monitoring patients with systemic lupus erythematosus in clinical practice and in observational studies. Ann Rheum Dis. 2010;69(7):1269–74.
Lane RJM, Mastaglia FL. Drug-induced myopathies in man. Lancet. 1978;312(8089):562–6.
Schakman O et al. Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell Biol. 2013;45(10):2163–72.
Jover JA et al. Combined treatment of giant-cell arteritis with methotrexate and prednisone, a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;134(2):106–14.
Mazlumzadeh M et al. Treatment of giant cell arteritis using induction therapy with high-dose glucocorticoids: a double-blind, placebo-controlled, randomized prospective clinical trial. Arthritis Rheum. 2006;54(10):3310–8.
Spiera RF et al. A prospective, double-blind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA). Clin Exp Rheumatol. 2001;19(5):495–501.
Gabriel SE et al. Adverse outcomes of antiinflammatory therapy among patients with polymyalgia rheumatica. Arthritis Rheum. 1997;40(10):1873–8.
Khaleeli AA et al. Corticosteroid myopathy: a clinical and pathological study. Clin Endocrinol. 1983;18(2):155–66.
Levin OS et al. Steroid myopathy in patients with chronic respiratory diseases. J Neurol Sci. 2014;338(1–2):96–101.
Hosono, O., et al. Quantitative analysis of skeletal muscle mass in patients with rheumatic diseases under glucocorticoid therapy—comparison among bioelectrical impedance analysis, computed tomography, and magnetic resonance imaging. Modern Rheumatology, 2014. 25(2):1–7.
Batchelor TT et al. Steroid myopathy in cancer patients. Neurology. 1997;48(5):1234–8.
Pereira RMR, de Carvalho JF. Glucocorticoid-induced myopathy. Joint Bone Spine. 2011;78(1):41–4.
LaPier TK. Glucocorticoid-induced muscle atrophy. The role of exercise in treatment and prevention. J Cardpulm Rehabil. 1997;17(2):76–84.
Horber FF et al. Evidence that prednisone-induced myopathy is reversed by physical training. J Clin Endocrinol Metab. 1985;61(1):83–8.
Hansen M et al. A randomised trial of differentiated prednisolone treatment in active rheumatoid arthritis. Clinical benefits and skeletal side effects. Ann Rheum Dis. 1999;58(11):713–8.
Laan RF et al. Low-dose prednisone induces rapid reversible axial bone loss in patients with rheumatoid arthritis. A randomized, controlled study. Ann Intern Med. 1993;119(10):963–8.
Nikitovic M et al. Direct health-care costs attributed to hip fractures among seniors: a matched cohort study. Osteoporos Int. 2013;24(2):659–69.
Rizzoli, R. and E. Biver, Glucocorticoid-induced osteoporosis: who to treat with what agent? Nat Rev Rheumatol, 2014.
Compston J et al. Diagnosis and management of osteoporosis in postmenopausal women and older men in the UK: National Osteoporosis Guideline Group (NOGG) update 2013. Maturitas. 2013;75(4):392–6. Provides guidelines for the management of osteoporosis.
Grossman JM et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res. 2010;62(11):1515–26.
Weinstein RS. Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med. 2011;365(1):62–70.
Ton FN et al. Effects of low-dose prednisone on bone metabolism. J Bone Miner Res. 2005;20(3):464–70.
den Uyl D, Bultink IE, Lems WF. Advances in glucocorticoid-induced osteoporosis. Curr Rheumatol Rep. 2011;13(3):233–40.
Hofbauer LC, Rauner M. Minireview: live and let die: molecular effects of glucocorticoids on bone cells. Mol Endocrinol. 2009;23(10):1525–31.
Albaum JM et al. Osteoporosis management among chronic glucocorticoid users: a systematic review. J Popul Ther Clin Pharmacol. 2014;21(3):e486–504.
Kadmiel M, Cidlowski JA. Glucocorticoid receptor signaling in health and disease. Trends Pharmacol Sci. 2013;34(9):518–30.
Lansang MC, Hustak LK. Glucocorticoid-induced diabetes and adrenal suppression: how to detect and manage them. Cleve Clin J Med. 2011;78(11):748–56.
Unizony, S., et al. Inpatient complications in patients with giant cell arteritis: decreased mortality and increased risk of thromboembolism, delirium and adrenal insufficiency. Rheumatology (Oxford), 2015.
Jamilloux Y et al. Recovery of adrenal function after long-term glucocorticoid therapy for giant cell arteritis: a cohort study. PLoS ONE. 2013;8(7):e68713.
Kirwan JR et al. The effect of therapeutic glucocorticoids on the adrenal response in a randomized controlled trial in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54(5):1415–21.
Duru, N., et al. EULAR evidence-based and consensus-based recommendations on the management of medium to high-dose glucocorticoid therapy in rheumatic diseases. Annals of the Rheumatic Diseases, 2013: 1–9. Provides EULAR recommendations for managing patients on medium-high-dose glucocorticoid therapy.
Gruvstad E et al. Comparison of methods for evaluation of the suppressive effects of prednisolone on the HPA axis and bone turnover: changes in s-DHEAS are as sensitive as the ACTH test. Int J Clin Pharmacol Ther. 2014;52(1):15–26. doi:10.5414/CP201938.
Kazlauskaite R et al. Corticotropin tests for hypothalamic-pituitary-adrenal insufficiency: a metaanalysis. J Clin Endocrinol Metab. 2008;93(11):4245–53.
Liu D et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9(1):30. Provides practical guidance for clinicians for monitoring and managing glucocorticoid-related adverse effects.
Ferris HA, Kahn CR. New mechanisms of glucocorticoid-induced insulin resistance: make no bones about it. J Clin Invest. 2012;122(11):3854–7.
Mazziotti G, Gazzaruso C, Giustina A. Diabetes in Cushing syndrome: basic and clinical aspects. Trends Endocrinol Metab. 2011;22(12):499–506.
Nwaneri, C., H. Cooper, and D. Bowen-Jones, Mortality in type 2 diabetes mellitus: magnitude of the evidence from a systematic review and meta-analysis. The British Journal of Diabetes & Vascular Disease, 2013.
Wassenberg S et al. Very low-dose prednisolone in early rheumatoid arthritis retards radiographic progression over two years: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 2005;52(11):3371–80.
van Everdingen AA et al. Low-dose prednisone therapy for patients with early active rheumatoid arthritis: clinical efficacy, disease-modifying properties, and side effects: a randomized, double-blind, placebo-controlled clinical trial. Ann Intern Med. 2002;136(1):1–12.
Ha Y et al. Glucocorticoid-induced diabetes mellitus in patients with systemic lupus erythematosus treated with high-dose glucocorticoid therapy. Lupus. 2011;20(10):1027–34.
Dasgupta B et al. BSR and BHPR guidelines for the management of polymyalgia rheumatica. Rheumatology (Oxford). 2010;49(1):186–90.
Dasgupta B et al. BSR and BHPR guidelines for the management of giant cell arteritis. Rheumatology (Oxford). 2010;49(8):1594–7.
Hancock AT et al. Risk of vascular events in patients with polymyalgia rheumatica. CMAJ. 2014;186(13):E495–501.
Uddhammar A et al. Increased mortality due to cardiovascular disease in patients with giant cell arteritis in northern Sweden. J Rheumatol. 2002;29(4):737–42.
Aviña-Zubieta JA et al. Immediate and past cumulative effects of oral glucocorticoids on the risk of acute myocardial infarction in rheumatoid arthritis: a population-based study. Rheumatology. 2013;52(1):68–75.
Mazzantini M et al. Adverse events during long-term low-dose glucocorticoid treatment of polymyalgia rheumatica: a retrospective study. J Rheumatol. 2012;39(3):552–7.
Hafström I et al. Effects of low-dose prednisolone on endothelial function, atherosclerosis, and traditional risk factors for atherosclerosis in patients with rheumatoid arthritis—a randomized study. J Rheumatol. 2007;34(9):1810–6.
Hafner F et al. Endothelial function and carotid intima-media thickness in giant-cell arteritis. Eur J Clin Investig. 2014;44(3):249–56.
Souverein PC et al. Use of oral glucocorticoids and risk of cardiovascular and cerebrovascular disease in a population based case-control study. Heart. 2004;90(8):859–65.
Tomasson G et al. Risk for cardiovascular disease early and late after a diagnosis of giant-cell arteritis: a cohort study. Ann Intern Med. 2014;160(2):73–80.
Kirwan J et al. A randomised placebo controlled 12 week trial of budesonide and prednisolone in rheumatoid arthritis. Ann Rheum Dis. 2004;63(6):688–95.
Bakker MF et al. Low-dose prednisone inclusion in a methotrexate-based, tight control strategy for early rheumatoid arthritis: a randomized trial. Ann Intern Med. 2012;156(5):329–39.
Hoes JN et al. Adverse events of low- to medium-dose oral glucocorticoids in inflammatory diseases: a meta-analysis. Ann Rheum Dis. 2009;68(12):1833–8.
Reynolds RM et al. Glucocorticoid treatment and impaired mood, memory and metabolism in people with diabetes: the Edinburgh type 2 diabetes study. Eur J Endocrinol. 2012;166(5):861–8.
Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361–7.
Ninan J et al. Mortality in patients with biopsy-proven giant cell arteritis: a south Australian population-based study. J Rheumatol. 2011;38(10):2215–7.
Nadkarni S et al. Investigational analysis reveals a potential role for neutrophils in giant-cell arteritis disease progression. Circ Res. 2014;114(2):242–8.
Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095–108.
Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21(2):271–92.
Harrison MJ et al. The validity and responsiveness of generic utility measures in rheumatoid arthritis: a review. J Rheumatol. 2008;35(4):592–602.
Buitinga L et al. Comparative responsiveness of the EuroQol-5D and short form 6D to improvement in patients with rheumatoid arthritis treated with tumor necrosis factor blockers: results of the Dutch Rheumatoid Arthritis Monitoring registry. Arthritis Care Res. 2012;64(6):826–32.
Gaujoux-Viala C et al. Responsiveness of EQ-5D and SF-6D in patients with early arthritis: results from the ESPOIR cohort. Ann Rheum Dis. 2012;71(9):1478–83.
Marra CA et al. A comparison of generic, indirect utility measures (the HUI2, HUI3, SF-6D, and the EQ-5D) and disease-specific instruments (the RAQoL and the HAQ) in rheumatoid arthritis. Soc Sci Med. 2005;60(7):1571–82.
Moller A, Sartipy U. Long-term health-related quality of life following surgery for lung cancer. Eur J Cardiothorac Surg. 2012;41(2):362–7. Demonstrates EQ-5D is responsive to AEs.
Brazier J, Tsuchiya A. Preference-based condition-specific measures of health: what happens to cross programme comparability? Health Econ. 2010;19(2):125–9.
NICE, Guide to the methods of technology appraisal 2013. Process and methods guides. 2013: London, UK.
Heather EM et al. Including adverse drug events in economic evaluations of anti-tumour necrosis factor-alpha drugs for adult rheumatoid arthritis: a systematic review of economic decision analytic models. Pharmacoeconomics. 2014;32(2):109–34.
Compliance with Ethics Guidelines
Conflict of Interest
Emma Harris, Ana Tiganescu, Sandy Tubeuf and Sarah Louise Mackie declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Health Economics and Quality of Life
Rights and permissions
About this article
Cite this article
Harris, E., Tiganescu, A., Tubeuf, S. et al. The Prediction and Monitoring of Toxicity Associated with Long-Term Systemic Glucocorticoid Therapy. Curr Rheumatol Rep 17, 38 (2015). https://doi.org/10.1007/s11926-015-0513-4
Published:
DOI: https://doi.org/10.1007/s11926-015-0513-4