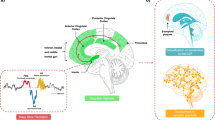
Abstract
Purpose of Review
In this review, we aim to integrate the most recent research highlighting alterations in sleep slow-wave activity (SWA), and impairments in neuroplasticity in major depressive disorder (MDD) into a novel model of disorder maintenance.
Recent Findings
Sleep homeostasis has been shown to be impaired in MDD, with a subset of individuals also demonstrating impaired SWA. SWA is considered a marker of the homeostatic regulation of sleep, and is implicated in the downscaling of synaptic strength in the context of maintaining homeostatic plasticity. Individuals with MDD have been shown to exhibit impairments in both neural plasticity such as loss of dendritic branching, and synaptic plasticity such as decreased long-term potentiation-dependent learning and memory.
Summary
Alterations in the homeostatic regulation of sleep, SWA, and synaptic plasticity in MDD suggest an underlying impairment in the modulation of synaptic strength. One candidate mechanism for this impairment is AMPA receptor trafficking.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Baglioni C, Nanovska S, Regen W, Spiegelhalder K, Feige B, Nissen C, et al. Sleep and mental disorders: a meta-analysis of polysomnographic research. Psychol Bull. 2016;142(9):969–90.
Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry. 2005;66(10):1254–69.
Armitage R, Hoffmann RF. Sleep EEG, depression and gender. Sleep Med Rev. 2001;5(3):237–46.
Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: a meta-analysis. Arch Gen Psychiatry. 1992;49(8):651–68.
Swanson LM, Hoffmann R, Armitage R. Sleep macroarchitecture in depression: sex differences. Open Sleep J. 2010;3:12–8.
Armitage R, Hoffmann R, Trivedi M, Rush AJ. Slow-wave activity in NREM sleep: sex and age effects in depressed outpatients and healthy controls. Psychiatry Res. 2000;95(3):201–13.
Goldschmied JR, Cheng P, Armitage R, Deldin PJ. Examining the effects of sleep delay on depressed males and females and healthy controls. J Sleep Res. 2014;23(6):664–72.
Frey S, Birchler-Pedross A, Hofstetter M, Brunner P, Götz T, Münch M, et al. Young women with major depression live on higher homeostatic sleep pressure than healthy controls. Chronobiol Int. 2012;29(3):278–94.
Duman RS, Voleti B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci. 2012;35(1):47–56.
•• Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62(2):143–50 This comprehensive theoretical review posits that the function of sleep is to facilitate the downscaling of synaptic strength in order to maintain synaptic homeostasis.
Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1(3):195–204.
Borbély AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51(5):483–93.
Werth E, Dijk DJ, Achermann P, Borbely AA. Dynamics of the sleep EEG after an early evening nap: experimental data and simulations. Am J Phys. 1996;271(3 Pt 2):R501–10.
Borbely AA, Wirz-Justice A. Sleep, sleep deprivation and depression. A hypothesis derived from a model of sleep regulation. Hum Neurobiol. 1982;1(3):205–10.
Boland EM, Rao H, Dinges DF, Smith RV, Goel N, Detre JA, et al. Meta-analysis of the antidepressant effects of acute sleep deprivation. J Clin Psychiatry. 2017;78(8):e1020–34.
Wirz-Justice A, Van den Hoofdakker RH. Sleep deprivation in depression: what do we know, where do we go? Biol Psychiatry. 1999;46(4):445–53.
Gillin JC, Buchsbaum M, Wu J, Clark C, Bunney W. Sleep deprivation as a model experimental antidepressant treatment: findings from functional brain imaging. Depress Anxiety. 2001;14(1):37–49.
Goldstein MR, Plante DT, Hulse BK, Sarasso S, Landsness EC, Tononi G, et al. Overnight changes in waking auditory evoked potential amplitude reflect altered sleep homeostasis in major depression. Acta Psychiatr Scand. 2012;125(6):468–77.
Plante DT, Goldstein MR, Landsness EC, Riedner BA, Guokas JJ, Wanger T, et al. Altered overnight modulation of spontaneous waking EEG reflects altered sleep homeostasis in major depressive disorder: a high-density EEG investigation. J Affect Disord. 2013;150(3):1167–73.
Kupfer DJ, Frank E, McEachran AB, Grochocinski VJ. Delta sleep ratio: a biological correlate of early recurrence in unipolar affective disorder. Arch Gen Psychiatry. 1990;47(12):1100–5.
Lotrich FE, Germain A. Decreased delta sleep ratio and elevated alpha power predict vulnerability to depression during interferon-alpha treatment. Acta Neuropsychiatr. 2015;27(01):14–24.
Thase ME, Fasiczka AL, Berman SR, Simons AD, Reynolds CF. Electroencephalographic sleep profiles before and after cognitive behavior therapy of depression. Arch Gen Psychiatry. 1998;55(2):138–44.
• Goldschmied JR, Cheng P, Hoffmann R, Boland EM, Deldin PJ, Armitage R. Effects of slow-wave activity on mood disturbance in major depressive disorder. Psychol Med. 2018;1–7. This study demonstrates that a slower rate of SWA dissipation is associated with increased mood disturbance in depression, providing evidence that the mechanism responsible for the modulation of SWA is associated with mood regulation in depression.
Liu ZW, Faraguna U, Cirelli C, Tononi G, Gao XB. Direct evidence for wake-related increases and sleep-related decreases in synaptic strength in rodent cortex. J Neurosci. 2010;30(25):8671–5.
Diering GH, Nirujogi RS, Roth RH, Worley PF, Pandey A, Huganir RL. Homer1a drives homeostatic scaling-down of excitatory synapses during sleep. Science. 2017;355(6324):511–5.
• de Vivo L, Bellesi M, Marshall W, Bushong EA, Ellisman MH, Tononi G, et al. Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science. 2017;355(6324):507–10 This study provides evidence for the synaptic homeostasis hypothesis by objectively demonstrating structural changes associated with the downscaling of synapses following sleep.
•• Wolf E, Kuhn M, Normann C, Mainberger F, Maier JG, Maywald S, et al. Synaptic plasticity model of therapeutic sleep deprivation in major depression. Sleep Med Rev. 2016;30:53–62 This theoretical paper reviews the synaptic plasticity model of depression and the synaptic homeostasis hypothesis and introduces an integrative model that posits that depression is characterized by a deficiency in synaptic strength and sleep deprivation results in antidepressant effects by normalizing this deficiency.
Wu JC, Bunney WE. The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am J Psychiatry. 1990;147(1):14–21.
Landsness EC, Goldstein MR, Peterson MJ, Tononi G, Benca RM. Antidepressant effects of selective slow wave sleep deprivation in major depression: a high-density EEG investigation. J Psychiatr Res. 2011;45(8):1019–26.
• Cheng P, Goldschmied J, Casement M, Kim HS, Hoffmann R, Armitage R, et al. Reduction in delta activity predicted improved negative affect in major depressive disorder. Psychiatry Res. 2015;228(3):715–8 This study demonstrates that following an experimental slow-wave disruption paradigm, a reduction in SWA was associated with a decrease in self-reported negative affect in MDD, providing early evidence that SWA may be depressogenic.
Beersma DG, Van den Hoofdakker RH. Can non-REM sleep be depressogenic? J Affect Disord. 1992;24(2):101–8.
McIntyre RS, Cha DS, Soczynska JK, Woldeyohannes HO, Gallaugher LA, Kudlow P, et al. Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates, and treatment interventions. Depress Anxiety. 2013;30(6):515–27.
Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7(5):426–37.
Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33(1):88–109.
Drevets WC, Price JL, Simpson JR Jr, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–7.
Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, et al. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004;56(9):640–50.
Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116–27.
Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64(6):527–32.
• Player MJ, Taylor JL, Weickert CS, Alonzo A, Sachdev P, Martin D, et al. Neuroplasticity in depressed individuals compared with healthy controls. Neuropsychopharmacology. 2013;38(11):2101–8 This is one of the first studies to objectively demonstrate neuroplasticity impairments in MDD. Paired associative stimulation, a TMS-based approach, provides an in-vivo index of associative neuroplasticity.
•• Marsden W. Synaptic plasticity in depression: molecular, cellular and functional correlates. Prog Neuro-Psychopharmacol Biol Psychiatry. 2013;43:168–84 A clear and concise review of the neurobiological features associated with synaptic plasticity, and of the pre-clinical and clinical evidence that suggests that depression is characterized by alterations to these features.
Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22(3):238–49.
Normann C, Schmitz D, Fürmaier A, Döing C, Bach M. Long-term plasticity of visually evoked potentials in humans is altered in major depression. Biol Psychiatry. 2007;62(5):373–80.
Popoli M, Gennarelli M, Racagni G. Modulation of synaptic plasticity by stress and antidepressants. Bipolar Disord. 2002;4(3):166–82.
Duncan WC, Zarate CA. Ketamine, sleep, and depression: current status and new questions. Curr Psychiatry Rep. 2013;15(9):394.
Alt A, Nisenbaum ES, Bleakman D, Witkin JM. A role for AMPA receptors in mood disorders. Biochem Pharmacol. 2006;71(9):1273–88.
• Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481–6 Critical study that demonstrates that the mechanism of action of ketamine may not be mediated directly via NMDA receptor antagonism, but rather via increased AMPA receptor throughput.
•• Freudenberg F, Celikel T, Reif A. The role of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in depression: central mediators of pathophysiology and antidepressant activity? Neurosci Biobehav Rev. 2015;52:193–206 A comprehensive review of the clinical and pre-clinical evidence that suggests that AMPA receptors are altered in MDD, and that the modulation of AMPA receptors may be involved with the mechanism of action of antidepressant drugs.
Murthy VN. Synaptic plasticity: step-wise strengthening. Curr Biol. 1998;8(18):R650–3.
Koizumi T, Tani H, Nakajima S, Nagai N, Suzuki T, Mimura M, et al. T108. AMPA receptor subunit expression and receptor binding in patients with major depressive disorder: a systematic review of postmortem studies. Biol Psychiatry. 2018;83(9):S170.
Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’shea DJ, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477(7363):171–8.
Groch S, Wilhelm I, Diekelmann S, Born J. The role of REM sleep in the processing of emotional memories: evidence from behavior and event-related potentials. Neurobiol Learn Mem. 2013;99:1–9.
Allen NJ, Barres BA. Signaling between glia and neurons: focus on synaptic plasticity. Curr Opin Neurobiol. 2005;15(5):542–8.
Havekes R, Vecsey CG, Abel T. The impact of sleep deprivation on neuronal and glial signaling pathways important for memory and synaptic plasticity. Cell Signal. 2012;24(6):1251–60.
Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58(6):545–53.
Bowley MP, Drevets WC, Öngür D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry. 2002;52(5):404–12.
Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45(9):1085–98.
Ngo HV, Martinetz T, Born J, Mölle M. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron. 2013;78(3):545–53.
Besedovsky L, Ngo HV, Dimitrov S, Gassenmaier C, Lehmann R, Born J. Auditory closed-loop stimulation of EEG slow oscillations strengthens sleep and signs of its immune-supportive function. Nat Commun. 2017;8(1):1984.
Acknowledgements
The authors would like to thank Elaine M. Boland for her generous contribution in the editing of this manuscript. The editors would like to thank Dr. Enrique Baca-Garcia for taking the time to review this manuscript.
Funding
Preparation of this article was supported by National Institute of Heart, Lung, and Blood Grant T32 HL007713 to Jennifer R. Goldschmied.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jennifer R. Goldschmied declares no potential conflicts of interest.
Philip Gehrman is a section editor for Current Psychiatry Reports.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Sleep Disorders
Rights and permissions
About this article
Cite this article
Goldschmied, J.R., Gehrman, P. An Integrated Model of Slow-Wave Activity and Neuroplasticity Impairments in Major Depressive Disorder. Curr Psychiatry Rep 21, 30 (2019). https://doi.org/10.1007/s11920-019-1013-4
Published:
DOI: https://doi.org/10.1007/s11920-019-1013-4