Abstract
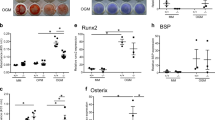
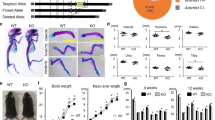
Thrombospondins (TSPs) are a family of five secreted multimeric matricellular proteins that share homology in the type II and III repeats and carboxy-terminal region. Type I repeats, also known as properdin or thrombospondin repeats (TSRs), are found in TSP1/2, but not TSP3-5. A variety of other secreted proteins contain TSRs, including the novel extracellular molecules, R-spondins. TSP family and many TSR-containing proteins, including R-spondins, are highly expressed in skeletal tissues during development and postnatal. TSP2 regulates the osteoblast lineage, influencing bone mass and geometry, as well as response to fracture healing, ovariectomy, and mechanical loading. Compound knockout mice of TSPs have revealed important mechanistic insights. TSP1/2 knockout mice have craniofacial dysmorphism, and TSP1/3/5 compound knockout mice display growth plate abnormalities. R-spondins promote osteoblast differentiation and R-spondin-2 deficiency results in skeletal developmental defects. Overall, TSP and other TSR molecules influence multiple aspects of bone development and remodeling.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance, •• Of major importance
Alford AI, Hankenson KD: Matricellular proteins: extracellular modulators of bone development, remodeling, and regeneration. Bone 206, 38:749–757.
Bornstein P: Thrombospondins as matricellular modulators of cell function. J Clin Invest 2001, 107:929–934.
Lawler J, Duquette M, Urry L, et al.: The evolution of the thrombospondin gene family. J Mol Evol 1993, 36:509–516.
Bornstein P, Sage EH: Thrombospondins. Methods Enzymol 1994, 245:62–85.
Adams JC: Thrombospondins: multifunctional regulators of cell interactions. Annu Rev Cell Dev Biol 2001, 17:25–51.
Bornstein P, Armstrong LC, Hankenson KD, et al.: Thrombospondin 2, a matricellular protein with diverse functions. Matrix Biol 2000, 19:557–568.
Lawler J: The functions of thrombospondin-1 and-2. Curr Opin Cell Biol 2000, 12:634–640.
Tucker RP: The thrombospondin type 1 repeat superfamily. Int J Biochem Cell Biol 2004, 36:969–974.
Tan K, Duquette M, Liu JH, et al.: Crystal structure of the TSP-1 type 1 repeats: a novel layered fold and its biological implication. J Cell Biol 2002, 159:373–382.
Crawford SE, Stellmach V, Murphy-Ullrich JE, et al.: Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell 1998, 93:1159–1170.
Posey KL, Hecht JT: The role of cartilage oligomeric matrix protein (COMP) in skeletal disease. Curr Drug Targets 2008, 9:869–877.
Deere M, Sanford T, Ferguson HL, et al.: Identification of twelve mutations in cartilage oligomeric matrix protein (COMP) in patients with pseudoachondroplasia. Am J Med Genet 1998, 80:510–513.
Kim KA, Zhao J, Andarmani S, et al.: R-Spondin proteins: a novel link to beta-catenin activation. Cell Cycle 2006, 5:23–26.
Iruela-Arispe ML, Liska DJ, Sage EH, et al.: Differential expression of thrombospondin 1, 2, and 3 during murine development. Dev Dyn 1993, 197:40–56.
Kyriakides TR, Zhu YH, Yang Z, et al.: The distribution of the matricellular protein, thrombospondin 2, in tissues of embryonic and adult mice. J Histochem Cytochem 1998, 46:1007–1015.
• Taylor DK, Meganck JA, Terkhorn S, et al.: Thrombospondin-2 influences the proportion of cartilage and bone during fracture healing. J Bone Miner Res 2009, 24:1043–1054. This study shows that the absence of TSP2 results in alterations in cartilage and bone content in fracture healing.
• Alford AI, Terkhorn SP, Reddy AB, Hankenson KD: Thrombospondin-2 regulates matrix mineralization in MC3T3-E1 pre-osteoblasts. Bone 2010, 46:464–471. This study shows that TSP2 regulates osteoblastogenesis independent of effects on proliferation.
Delany A, Hankenson K: Thrombospondin-2 and SPARC/osteonectin are critical regulators of bone remodeling. J Cell Commun Signal 2009, 3:227–238.
Riddle R, Khatri R, Schipani E, et al.: Role of hypoxia-inducible factor-1α in angiogenic–osteogenic coupling. J Mol Med 2009, 87:583–590.
• Meng H, Zhang X, Hankenson KD, et al.: Thrombospondin2 potentiates notch3/jagged1 signaling. J Biol Chem 2009, 284:7866–7874.
Engin F, Lee B: NOTCHing the bone: insights into multi-functionality. Bone 2010, 46:274–280.
Akemichi U, Yoshihiro M, Keiko M, et al.: Constitutive expression of thrombospondin 1 in MC3T3-E1 osteoblastic cells inhibits mineralization. J Cell Physiol 2006, 209:322–332.
Dalla-Torre C, Yoshimoto M, Lee CH, et al.: Effects of THBS3, SPARC and SPP1 expression on biological behavior and survival in patients with osteosarcoma. BMC Cancer 2006, 6:237.
Hankenson KD, Hormuzdi SG, Meganck JA, et al.: Mice with a disruption of the thrombospondin 3 gene differ in geometric and biomechanical properties of bone and have accelerated development of the femoral head. Mol Cell Biol 2005, 25:5599–5606.
• Posey KL, Hankenson K, Veerisetty AC, et al.: Skeletal abnormalities in mice lacking extracellular matrix proteins, thrombospondin-1, thrombospondin-3, thrombospondin-5, and type IX collagen. Am J Pathol 2008, 172:1664–1674. This study showed significant defects in growth cartilage in compound TSP1/3/5 knockout mice.
Fang C, Carlson CS, Leslie MP, et al.: Molecular cloning, sequencing, and tissue and developmental expression of mouse cartilage oligomeric matrix protein (COMP). J Orthop Res 2000, 18:593–603.
Posey KL, Davies S, Bales ES, et al.: In vivo human Cartilage oligomeric matrix protein (COMP) promoter activity. Matrix Biol 2005, 24:539–549.
Urakami T, Manki A, Inoue T, et al.: Clinical significance of decreased serum concentration of cartilage oligomeric matrix protein in systemic juvenile idiopathic arthritis. J. Rheumatol 2006, 33:996–1000.
Koelling S, Clauditz TS, Kaste M, et al.: Cartilage oligomeric matrix protein is involved in human limb development and in the pathogenesis of osteoarthritis. Arthritis Res Ther 2006, 8:R56.
Mann HH, Ozbek S, Engel J, et al.: Interactions between the cartilage oligomeric matrix protein and matrilins. Implications for matrix assembly and the pathogenesis of chondrodysplasias. J Biol Chem 2004, 279:25294–25298.
Hecht JT, Nelson LD, Crowder E, et al.: Mutations in exon 17B of cartilage oligomeric matrix protein (COMP) cause pseudoachondroplasia. Nat Genet 1995, 10:325–329.
Horton WA, Hecht JT: The chondrodysplasias. In Connective Tissue and Its Heritable Disorders. Molecular, Genetic and Medical Aspects, edn 2. New York: Wiley-Liss; 2001:614–675.
Hecht JT, Montufar-Solis D, Decker G, et al.: Retention of cartilage oligomeric matrix protein (COMP) and cell death in redifferentiated pseudoachondroplasia chondrocytes. Matrix Biol 1998, 17:625–633.
Posey KL, Hayes E, Haynes R, et al.: Role of TSP-5/COMP in pseudoachondroplasia. Int J Biochem Cell Biol 2004, 36:1005–1012.
Merritt TM, Bick R, Poindexter BJ, et al.: Unique matrix structure in the rough endoplasmic reticulum cisternae of pseudoachondroplasia chondrocytes. Am J Pathol 2007, 170:293–300.
Svensson L, Aszodi A, Heinegard D, et al.: Cartilage oligomeric matrix protein-deficient mice have normal skeletal development. Mol Cell Biol 2002, 22:4366–4371.
•• Posey KL, Veerisetty AC, Liu P, et al.: An inducible cartilage oligomeric matrix protein mouse model recapitulates human pseudoachondroplasia phenotype. Am J Pathol 2009, 175:1555–1563. This seminal study shows that a mouse model of a TSP5 mutation recapitulates the human PSACH condition.
Chen FH, Herndon ME, Patel N, et al.: Interaction of cartilage oligomeric matrix protein/thrombospondin 5 with aggrecan. J Biol Chem 2007, 282:24591–24598.
Kipnes J, Carlberg AL, Loredo GA, et al.: Effect of cartilage oligomeric matrix protein on mesenchymal chondrogenesis in vitro. Osteoarthritis Cartilage 2003, 11:442–454.
Xu K, Zhang Y, Ilalov K, et al.: Cartilage oligomeric matrix protein associates with granulin-epithelin precursor (GEP) and potentiates GEP-stimulated chondrocyte proliferation. J Biol Chem 2007, 282:11347–11355.
Chen FH, Thomas AO, Hecht JT, et al.: Cartilage oligomeric matrix protein/thrombospondin 5 supports chondrocyte attachment through interaction with integrins. J Biol Chem 2005, 280:32655–32661.
Holden P, Meadows RS, Chapman KL, et al.: Cartilage oligomeric matrix protein interacts with type IX collagen, and disruptions to these interactions identify a pathogenetic mechanism in a bone dysplasia family. J Biol Chem 2001, 276:6046–6055.
Thur J, Rosenberg K, Nitsche DP, et al.: Mutations in cartilage oligomeric matrix protein causing pseudoachondroplasia and multiple epiphyseal dysplasia affect binding of calcium and collagen I, II, and IX. J Biol Chem 2001, 276:6083–6092.
Halasz K, Kassner A, Morgelin M, et al.: COMP acts as a catalyst in collagen fibrillogenesis. J Biol Chem 2007, 282:31166–31173.
Hecht JT, Hayes E, Haynes R, et al.: COMP mutations, chondrocyte function and cartilage matrix. Matrix Biol 2005, 23:525–533.
Kamata T, Katsube K, Michikawa M, et al.: R-spondin, a novel gene with thrombospondin type 1 domain, was expressed in the dorsal neural tube and affected in Wnts mutants. Biochim Biophys Acta 2004, 1676:51–62.
Kim KA, Wagle M, Tran K, et al.: R-spondin family members regulate the Wnt pathway by a common mechanism. Mol Biol Cell 2008, 19:2588–2596.
Bell SM, Schreiner CM, Wert SE, et al.: R-spondin 2 is required for normal laryngeal-tracheal, lung and limb morphogenesis. Development 2008, 135:1049–1058.
Nam JS, Turcotte TJ, Yoon JK: Dynamic expression of R-spondin family genes in mouse development. Gene Expr Patterns 2007, 7:306–312.
Chen Y, Alman BA: Wnt pathway, an essential role in bone regeneration. J Cell Biochem 2009, 106:353–362.
Aoki M, Kiyonari H, Nakamura H, et al.: R-spondin2 expression in the apical ectodermal ridge is essential for outgrowth and patterning in mouse limb development. Dev Growth Differ 2008, 50:85–95.
Nam JS, Park E, Turcotte TJ, et al.: Mouse R-spondin2 is required for apical ectodermal ridge maintenance in the hindlimb. Dev Biol 2007, 311:124–135.
Yamada W, Nagao K, Horikoshi K, et al.: Craniofacial malformation in R-spondin2 knockout mice. Biochem Biophys Res Commun 2009, 381:453–458.
Blaydon DC, Ishii Y, O'Toole EA, et al.: The gene encoding R-spondin 4 (RSPO4), a secreted protein implicated in Wnt signaling, is mutated in inherited anonychia. Nat Genet 2006, 38:1245–1247.
Friedman MS, Oyserman SM, Hankenson KD: Wnt11 promotes osteoblast maturation and mineralization through R-spondin 2. J Biol Chem 2009, 284:14117–14125.
• Lu W, Kim KA, Liu J, et al.: R-spondin1 synergizes with Wnt3A in inducing osteoblast differentiation and osteoprotegerin expression. FEBS Lett 2008, 582:643–650. This study shows that R-spondin-2 is a regulator of BMP-induced osteoblast differentiation.
Disclosure
Dr. Hankenson is supported by funding from the National Institutes of Health (NIH) (R01 AR054714, R01 AR049682, and R01 DE017471), and by the NIH-funded (P30 AR050950) Penn Center for Musculoskeletal Disorders. Dr. Sweetwyne is supported by NIH grant K12-GM081259. No other potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hankenson, K.D., Sweetwyne, M.T., Shitaye, H. et al. Thrombospondins and Novel TSR-containing Proteins, R-spondins, Regulate Bone Formation and Remodeling. Curr Osteoporos Rep 8, 68–76 (2010). https://doi.org/10.1007/s11914-010-0017-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-010-0017-0