Abstract
Purpose of Review
Primary age-related tauopathy (PART) was recently proposed as a pathologic diagnosis for brains that harbor neurofibrillary tangles (Braak stage ≤ 4) with little, if any, amyloid burden. We sought to review the clinicopathologic findings related to PART.
Recent Findings
Most adult human brains show at least focal tauopathic changes, and the majority of individuals with PART do not progress to dementia. Older age and cognitive impairment correlate with increased Braak stage, and multivariate analyses suggest that the rate of cognitive decline is less than matched patients with Alzheimer disease (AD). It remains unclear whether PART is a distinct tauopathic entity separate from AD or rather represents an earlier histologic stage of AD.
Summary
Cognitive decline in PART is usually milder than AD and correlates with tauopathic burden. Biomarker and ligand-based radiologic studies will be important to define PART antemortem and prospectively follow its natural history.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
den Dunnen WF, Brouwer WH, Bijlard E, Kamphuis J, van Linschoten K, Eggens-Meijer E, et al. No disease in the brain of a 115-year-old woman. Neurobiol Aging. 2008;29(8):1127–32. https://doi.org/10.1016/j.neurobiolaging.2008.04.010.
Hickman RA, Faustin A, Wisniewski T. Alzheimer disease and its growing epidemic: risk factors, biomarkers, and the urgent need for therapeutics. Neurol Clin. 2016;34(4):941–53. https://doi.org/10.1016/j.ncl.2016.06.009.
James BD, Bennett DA. Causes and patterns of dementia: an update in the era of redefining Alzheimer’s disease. Annu Rev Public Health. 2019;40:65–84. https://doi.org/10.1146/annurev-publhealth-040218-043758.
Rabinovici GD. Late-onset Alzheimer disease. Continuum (Minneap Minn). 2019;25(1):14–33. https://doi.org/10.1212/con.0000000000000700.
Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet. 2001;357(9251):169–75. https://doi.org/10.1016/s0140-6736(00)03589-3.
Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62(11):1087–95. https://doi.org/10.1093/jnen/62.11.1087.
Elobeid A, Libard S, Leino M, Popova SN, Alafuzoff I. Altered proteins in the aging brain. J Neuropathol Exp Neurol. 2016;75(4):316–25. https://doi.org/10.1093/jnen/nlw002.
Tomlinson BE, Blessed G, Roth M. Observations on the brains of non-demented old people. J Neurol Sci. 1968;7(2):331–56. https://doi.org/10.1016/0022-510x(68)90154-8.
Ganz AB, Beker N, Hulsman M, Sikkes S, Netherlands Brain B, Scheltens P, et al. Neuropathology and cognitive performance in self-reported cognitively healthy centenarians. Acta Neuropathol Commun. 2018;6(1):64. https://doi.org/10.1186/s40478-018-0558-5.
Mizutani T, Shimada H. Neuropathological background of twenty-seven centenarian brains. J Neurol Sci. 1992;108(2):168–77.
Itoh Y, Yamada M, Suematsu N, Matsushita M, Otomo E. An immunohistochemical study of centenarian brains: a comparison. J Neurol Sci. 1998;157(1):73–81.
Wood JG, Mirra SS, Pollock NJ, Binder LI. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau). Proc Natl Acad Sci. 1986;83(11):4040–3.
Long JM, Holtzman DM. Alzheimer disease: an update on pathobiology and treatment strategies. Cell. 2019;179(2):312–39. https://doi.org/10.1016/j.cell.2019.09.001.
Braak H, Del Tredici K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 2011;121(2):171–81. https://doi.org/10.1007/s00401-010-0789-4.
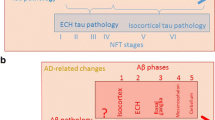
•• Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70(11):960–9. https://doi.org/10.1097/NEN.0b013e318232a379Although nearly a decade old, this monumental paper describes the frequency and distribution of tauopathic changes and amyloid burden in over 2300 brains ranging from infancy to 100 years of age. This work demonstrated that amyloid deposition occurs at a much later time point than the onset of tauopathic changes.
Grinberg LT, Rub U, Ferretti RE, Nitrini R, Farfel JM, Polichiso L, et al. The dorsal raphe nucleus shows phospho-tau neurofibrillary changes before the transentorhinal region in Alzheimer’s disease. A precocious onset? Neuropathol Appl Neurobiol. 2009;35(4):406–16. https://doi.org/10.1111/j.1365-2990.2009.00997.x.
Elobeid A, Soininen H, Alafuzoff I. Hyperphosphorylated tau in young and middle-aged subjects. Acta Neuropathol. 2012;123(1):97–104. https://doi.org/10.1007/s00401-011-0906-z.
Attems J, Thomas A, Jellinger K. Correlations between cortical and subcortical tau pathology. Neuropathol Appl Neurobiol. 2012;38(6):582–90. https://doi.org/10.1111/j.1365-2990.2011.01244.x.
Kovacs GG, Milenkovic I, Wohrer A, Hoftberger R, Gelpi E, Haberler C, et al. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta Neuropathol. 2013;126(3):365–84. https://doi.org/10.1007/s00401-013-1157-y.
Bancher C, Leitner H, Jellinger K, Eder H, Setinek U, Fischer P, et al. On the relationship between measles virus and Alzheimer neurofibrillary tangles in subacute sclerosing panencephalitis. Neurobiol Aging. 1996;17(4):527–33. https://doi.org/10.1016/0197-4580(96)00069-3.
Wisniewski K, Jervis GA, Moretz RC, Wisniewski HM. Alzheimer neurofibrillary tangles in diseases other than senile and presenile dementia. Ann Neurol. 1979;5(3):288–94. https://doi.org/10.1002/ana.410050311.
McKee AC, Stein TD, Kiernan PT, Alvarez VE. The neuropathology of chronic traumatic encephalopathy. Brain Pathol (Zurich, Switzerland). 2015;25(3):350–64. https://doi.org/10.1111/bpa.12248.
Auer IA, Schmidt ML, Lee VM, Curry B, Suzuki K, Shin RW, et al. Paired helical filament tau (PHFtau) in Niemann-Pick type C disease is similar to PHFtau in Alzheimer’s disease. Acta Neuropathol. 1995;90(6):547–51. https://doi.org/10.1007/bf00318566.
Turk KW, Budson AE. Chronic traumatic encephalopathy. Continuum (Minneap Minn). 2019;25(1):187–207. https://doi.org/10.1212/con.0000000000000686.
Itoh, Yamada M, Yoshida R, Suematsu N, Oka T, Matsushita M, et al. Dementia characterized by abundant neurofibrillary tangles and scarce senile plaques: a quantitative pathological study. Eur Neurol. 1996;36(2):94–7. https://doi.org/10.1159/000117216.
Jellinger KA, Bancher C. Senile dementia with tangles (tangle predominant form of senile dementia). Brain Pathol. 1998;8(2):367–76. https://doi.org/10.1111/j.1750-3639.1998.tb00160.x.
Ulrich J. Abundant neurofibrillary tangles without senile plaques in a subset of patients with senile dementia. Neurodegeneration. 1992;1:257–64.
Bancher C, Jellinger KA. Neurofibrillary tangle predominant form of senile dementia of Alzheimer type: a rare subtype in very old subjects. Acta Neuropathol. 1994;88(6):565–70. https://doi.org/10.1007/bf00296494.
Yamada M. Senile dementia of the neurofibrillary tangle type (tangle-only dementia): neuropathological criteria and clinical guidelines for diagnosis. Neuropathology. 2003;23(4):311–7. https://doi.org/10.1046/j.1440-1789.2003.00522.x.
Ikeda K, Akiyama H, Arai T, Oda T, Kato M, Iseki E, et al. Clinical aspects of ‘senile dementia of the tangle type’-- a subset of dementia in the senium separable from late-onset Alzheimer’s disease. Dement Geriatr Cogn Disord. 1999;10(1):6–11. https://doi.org/10.1159/000017091.
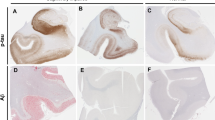
•• Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128(6):755–66. https://doi.org/10.1007/s00401-014-1349-0This consensus paper introduced the concept of PART and outlined the guidelines/criteria for rendering the diagnosis.
Jellinger K, Attems J. Neurofibrillary tangle-predominant dementia: comparison with classical Alzheimer disease. Acta Neuropathol. 2007;113(2):107–17.
Quintas-Neves M, Teylan MA, Besser L, Soares-Fernandes J, Mock CN, Kukull WA, et al. Magnetic resonance imaging brain atrophy assessment in primary age-related tauopathy (PART). Acta Neuropathol Commun. 2019;7(1):204. https://doi.org/10.1186/s40478-019-0842-z.
Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–59. https://doi.org/10.1007/bf00308809.
•• Josephs KA, Murray ME, Tosakulwong N, Whitwell JL, Knopman DS, Machulda MM, et al. Tau aggregation influences cognition and hippocampal atrophy in the absence of beta-amyloid: a clinico-imaging-pathological study of primary age-related tauopathy (PART). Acta Neuropathol. 2017;133(5):705–15. https://doi.org/10.1007/s00401-017-1681-2A clinicopathologic study of definite PART that incorporated both pathology, neuropsychologic testing, and neuroimaging. The authors describe correlation of Braak stage with cognitive impairment and show an association between semantic memory impairment and anterior hippocampal atrophy.
• Jellinger KA. Different patterns of hippocampal tau pathology in Alzheimer’s disease and PART. Acta Neuropathol. 2018. https://doi.org/10.1007/s00401-018-1894-zThis letter outlines an important pathologic difference in the neuropathology of PART and AD that the tauopathic burden is greater in CA2 than CA1, contrary to what is usually seen in classical AD.
McKee AC, Cairns NJ, Dickson DW, Folkerth RD, Keene CD, Litvan I, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016;131(1):75–86.
Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225(4667):1168–70.
Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791–800. https://doi.org/10.1212/wnl.58.12.1791.
•• Duyckaerts C, Braak H, Brion J-P, Buée L, Del Tredici K, Goedert M, et al. PART is part of Alzheimer disease. Acta Neuropathol. 2015;129(5):749–56. https://doi.org/10.1007/s00401-015-1390-7This article puts forth the argument and provides evidence to suggest that PART and AD rest on a spectrum and that PART is an earlier stage of AD.
Besser LM, Mock C, Teylan MA, Hassenstab J, Kukull WA, Crary JF. Differences in cognitive impairment in primary age-related Tauopathy versus Alzheimer disease. J Neuropathol Exp Neurol. 2019;78(3):219–28.
Neltner JH, Abner EL, Jicha GA, Schmitt FA, Patel E, Poon LW, et al. Brain pathologies in extreme old age. Neurobiol Aging. 2016;37:1–11.
Kovacs GG, Alafuzoff I, Al-Sarraj S, Arzberger T, Bogdanovic N, Capellari S, et al. Mixed brain pathologies in dementia: the BrainNet Europe consortium experience. Dement Geriatr Cogn Disord. 2008;26(4):343–50. https://doi.org/10.1159/000161560.
Alafuzoff I. Alzheimer’s disease-related lesions. J Alzheimers Dis. 2013;33(Suppl 1):S173–9. https://doi.org/10.3233/jad-2012-129024.
Robinson JL, Lee EB, Xie SX, Rennert L, Suh E, Bredenberg C, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain J Neurol. 2018;141(7):2181–93. https://doi.org/10.1093/brain/awy146.
Kovacs GG, Ferrer I, Grinberg LT, Alafuzoff I, Attems J, Budka H, et al. Aging-related tau astrogliopathy (ARTAG): harmonized evaluation strategy. Acta Neuropathol. 2016;131(1):87–102. https://doi.org/10.1007/s00401-015-1509-x.
Ferrer I, Santpere G, van Leeuwen FW. Argyrophilic grain disease. Brain J Neurol. 2008;131(Pt 6):1416–32. https://doi.org/10.1093/brain/awm305.
Saito Y, Ruberu NN, Sawabe M, Arai T, Tanaka N, Kakuta Y, et al. Staging of argyrophilic grains: an age-associated tauopathy. J Neuropathol Exp Neurol. 2004;63(9):911–8. https://doi.org/10.1093/jnen/63.9.911.
Braak H, Braak E. Argyrophilic grain disease: frequency of occurrence in different age categories and neuropathological diagnostic criteria. J Neural Transm (Vienna, Austria : 1996). 1998;105(8–9):801–19. https://doi.org/10.1007/s007020050096.
Nelson PT, Trojanowski JQ, Abner EL, Al-Janabi OM, Jicha GA, Schmitt FA, et al. “New old pathologies”: AD, PART, and cerebral age-related TDP-43 with sclerosis (CARTS). J Neuropathol Exp Neurol. 2016;75(6):482–98.
Wennberg AM, Whitwell JL, Tosakulwong N, Weigand SD, Murray ME, Machulda MM, et al. The influence of tau, amyloid, alpha-synuclein, TDP-43, and vascular pathology in clinically normal elderly individuals. Neurobiol Aging. 2019;77:26–36. https://doi.org/10.1016/j.neurobiolaging.2019.01.008.
Arnold SJ, Dugger BN, Beach TG. TDP-43 deposition in prospectively followed, cognitively normal elderly individuals: correlation with argyrophilic grains but not other concomitant pathologies. Acta Neuropathol. 2013;126(1):51–7. https://doi.org/10.1007/s00401-013-1110-0.
Wilson AC, Dugger BN, Dickson DW, Wang DS. TDP-43 in aging and Alzheimer’s disease—a review. Int J Clin Exp Pathol. 2011;4(2):147–55.
Arai T, Mackenzie IR, Hasegawa M, Nonoka T, Niizato K, Tsuchiya K, et al. Phosphorylated TDP-43 in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol. 2009;117(2):125–36. https://doi.org/10.1007/s00401-008-0480-1.
McAleese KE, Walker L, Erskine D, Thomas AJ, McKeith IG, Attems J. TDP-43 pathology in Alzheimer’s disease, dementia with Lewy bodies and ageing. Brain Pathol (Zurich, Switzerland). 2017;27(4):472–9. https://doi.org/10.1111/bpa.12424.
Josephs KA, Murray ME, Whitwell JL, Parisi JE, Petrucelli L, Jack CR, et al. Staging TDP-43 pathology in Alzheimer’s disease. Acta Neuropathol. 2014;127(3):441–50. https://doi.org/10.1007/s00401-013-1211-9.
Josephs KA, Murray ME, Whitwell JL, Tosakulwong N, Weigand SD, Petrucelli L, et al. Updated TDP-43 in Alzheimer’s disease staging scheme. Acta Neuropathol. 2016;131(4):571–85. https://doi.org/10.1007/s00401-016-1537-1.
Zhang X, Sun B, Wang X, Lu H, Shao F, Rozemuller AJM, et al. Phosphorylated TDP-43 staging of primary age-related tauopathy. Neurosci Bull. 2019;35(2):183–92. https://doi.org/10.1007/s12264-018-0300-0.
Markesbery WR, Jicha GA, Liu H, Schmitt FA. Lewy body pathology in normal elderly subjects. J Neuropathol Exp Neurol. 2009;68(7):816–22. https://doi.org/10.1097/NEN.0b013e3181ac10a7.
Gibb WR. Idiopathic Parkinson’s disease and the Lewy body disorders. Neuropathol Appl Neurobiol. 1986;12(3):223–34. https://doi.org/10.1111/j.1365-2990.1986.tb00136.x.
Hamilton RL. Lewy bodies in Alzheimer’s disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol. 2000;10(3):378–84. https://doi.org/10.1111/j.1750-3639.2000.tb00269.x.
Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–204. https://doi.org/10.1212/01.wnl.0000271090.28148.24.
Kapasi A, Schneider JA. Vascular contributions to cognitive impairment, clinical Alzheimer’s disease, and dementia in older persons. Biochim Biophys Acta. 2016;1862(5):878–86. https://doi.org/10.1016/j.bbadis.2015.12.023.
Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer’s disease—lessons from pathology. BMC Med. 2014;12:206. https://doi.org/10.1186/s12916-014-0206-2.
Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Santacruz K, et al. Brains with medial temporal lobe neurofibrillary tangles but no neuritic amyloid plaques are a diagnostic dilemma but may have pathogenetic aspects distinct from Alzheimer disease. J Neuropathol Exp Neurol. 2009;68(7):774–84. https://doi.org/10.1097/NEN.0b013e3181aacbe9.
Jefferson-George KS, Wolk DA, Lee EB, McMillan CT. Cognitive decline associated with pathological burden in primary age-related tauopathy. Alzheimers Dement’. 2017;13(9):1048–53.
Besser LM, Crary JF, Mock C, Kukull WA. Comparison of symptomatic and asymptomatic persons with primary age-related tauopathy. Neurology. 2017;89(16):1707–15.
Bell WR, An Y, Kageyama Y, English C, Rudow GL, Pletnikova O, et al. Neuropathologic, genetic, and longitudinal cognitive profiles in primary age-related tauopathy (PART) and Alzheimer’s disease. Alzheimers Dement. 2019;15(1):8–16. https://doi.org/10.1016/j.jalz.2018.07.215.
Teylan M, Besser LM, Crary JF, Mock C, Gauthreaux K, Thomas NM, et al. Clinical diagnoses among individuals with primary age-related tauopathy versus Alzheimer’s neuropathology. Lab Investig. 2019;99(7):1049–55.
• Teylan M, Mock C, Gauthreaux K, Chen YC, Chan KCG, Hassenstab J, et al. Cognitive trajectory in mild cognitive impairment due to primary age-related tauopathy. Brain J Neurol. 2020;143(2):611–21. https://doi.org/10.1093/brain/awz403This paper compares individuals with PART and matched individuals with AD and shows a slower cognitive decline in PART than in AD.
Mock C, Teylan M, Beecham G, Besser L, Cairns NJ, Crary JF, et al. The utility of the National Alzheimer’s Coordinating Center’s Database for the rapid assessment of evolving neuropathologic conditions. Alzheimer Dis Assoc Disord. 2020;34:105–11. https://doi.org/10.1097/wad.0000000000000380.
Kryscio R, Abner E, Jicha G, Nelson P, Smith C, Van Eldik L, et al. Self-reported memory complaints: a comparison of demented and unimpaired outcomes. J Prev Alzheimer’s Dis. 2016;3(1):13.
Santa-Maria I, Haggiagi A, Liu X, Wasserscheid J, Nelson PT, Dewar K, et al. The MAPT H1 haplotype is associated with tangle-predominant dementia. Acta Neuropathol. 2012;124(5):693–704. https://doi.org/10.1007/s00401-012-1017-1.
Jack CR Jr, Knopman DS, Chetelat G, Dickson D, Fagan AM, Frisoni GB, et al. Suspected non-Alzheimer disease pathophysiology--concept and controversy. Nat Rev Neurol. 2016;12(2):117–24. https://doi.org/10.1038/nrneurol.2015.251.
Jack CR Jr, Knopman DS, Weigand SD, Wiste HJ, Vemuri P, Lowe V, et al. An operational approach to National Institute on Aging-Alzheimer’s Association criteria for preclinical Alzheimer disease. Ann Neurol. 2012;71(6):765–75. https://doi.org/10.1002/ana.22628.
Jack CR Jr, Wiste HJ, Weigand SD, Rocca WA, Knopman DS, Mielke MM, et al. Age-specific population frequencies of cerebral beta-amyloidosis and neurodegeneration among people with normal cognitive function aged 50-89 years: a cross-sectional study. Lancet Neurol. 2014;13(10):997–1005. https://doi.org/10.1016/s1474-4422(14)70194-2.
Burnham SC, Bourgeat P, Dore V, Savage G, Brown B, Laws S, et al. Clinical and cognitive trajectories in cognitively healthy elderly individuals with suspected non-Alzheimer’s disease pathophysiology (SNAP) or Alzheimer’s disease pathology: a longitudinal study. Lancet Neurol. 2016;15(10):1044–53. https://doi.org/10.1016/s1474-4422(16)30125-9.
Soldan A, Pettigrew C, Fagan AM, Schindler SE, Moghekar A, Fowler C, et al. ATN profiles among cognitively normal individuals and longitudinal cognitive outcomes. Neurology. 2019;92(14):e1567–e79. https://doi.org/10.1212/wnl.0000000000007248.
Caroli A, Prestia A, Galluzzi S, Ferrari C, van der Flier WM, Ossenkoppele R, et al. Mild cognitive impairment with suspected nonamyloid pathology (SNAP): prediction of progression. Neurology. 2015;84(5):508–15. https://doi.org/10.1212/wnl.0000000000001209.
Wisse LEM, Butala N, Das SR, Davatzikos C, Dickerson BC, Vaishnavi SN, et al. Suspected non-AD pathology in mild cognitive impairment. Neurobiol Aging. 2015;36(12):3152–62. https://doi.org/10.1016/j.neurobiolaging.2015.08.029.
Weigand AJ, Bangen KJ, Thomas KR, Delano-Wood L, Gilbert PE, Brickman AM, et al. Is tau in the absence of amyloid on the Alzheimer’s continuum?: A study of discordant PET positivity. Brain Commun. 2020;2(1):fcz046.
Price JL, Morris JC. Tangles and plaques in nondemented aging and "preclinical" Alzheimer’s disease. Ann Neurol. 1999;45(3):358–68. https://doi.org/10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x.
Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, et al. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12(3):292–323. https://doi.org/10.1016/j.jalz.2016.02.002.
Ehrenberg AJ, Nguy AK, Theofilas P, Dunlop S, Suemoto CK, Di Lorenzo Alho AT, et al. Quantifying the accretion of hyperphosphorylated tau in the locus coeruleus and dorsal raphe nucleus: the pathological building blocks of early Alzheimer’s disease. Neuropathol Appl Neurobiol. 2017;43(5):393–408. https://doi.org/10.1111/nan.12387.
Andres-Benito P, Fernandez-Duenas V, Carmona M, Escobar LA, Torrejon-Escribano B, Aso E, et al. Locus coeruleus at asymptomatic early and middle Braak stages of neurofibrillary tangle pathology. Neuropathol Appl Neurobiol. 2017;43(5):373–92. https://doi.org/10.1111/nan.12386.
Pires G, McElligott S, Drusinsky S, Halliday G, Potier MC, Wisniewski T, et al. Secernin-1 is a novel phosphorylated tau binding protein that accumulates in Alzheimer’s disease and not in other tauopathies. Acta Neuropathol Commun. 2019;7(1):195. https://doi.org/10.1186/s40478-019-0848-6.
Bancher C, Egensperger R, Kösel S, Jellinger K, Graeber MB. Low prevalence of apolipoprotein E ε4 allele in the neurofibrillary tangle predominant form of senile dementia. Acta Neuropathol. 1997;94(5):403–9. https://doi.org/10.1007/s004010050726.
Janocko NJ, Brodersen KA, Soto-Ortolaza AI, Ross OA, Liesinger AM, Duara R, et al. Neuropathologically defined subtypes of Alzheimer’s disease differ significantly from neurofibrillary tangle-predominant dementia. Acta Neuropathol. 2012;124(5):681–92. https://doi.org/10.1007/s00401-012-1044-y.
Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011;10(9):785–96. https://doi.org/10.1016/s1474-4422(11)70156-9.
Gibbons GS, Lee VMY, Trojanowski JQ. Mechanisms of cell-to-cell transmission of pathological tau: a review. JAMA Neurol. 2019;76(1):101–8. https://doi.org/10.1001/jamaneurol.2018.2505.
Esiri MM. Ageing and the brain. J Pathol. 2007;211(2):181–7. https://doi.org/10.1002/path.2089.
Acknowledgments
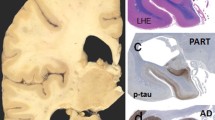
We thank Dr. Jean-Paul Vonsattel for useful comments and providing the macroscopic images of Fig. 1a, b. We extend our sincere gratitude to all of the patients, families, and caregivers for their generosity in brain donation for neurodegenerative research.
Funding
RAH is supported by grant funding from the Huntington Disease Society of America and Hereditary Disease Foundation. This review is supported by P50 AG008702 (PI Scott Small, MD) and P30AG066512 (TW).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Dementia
Rights and permissions
About this article
Cite this article
Hickman, R.A., Flowers, X.E. & Wisniewski, T. Primary Age-Related Tauopathy (PART): Addressing the Spectrum of Neuronal Tauopathic Changes in the Aging Brain. Curr Neurol Neurosci Rep 20, 39 (2020). https://doi.org/10.1007/s11910-020-01063-1
Published:
DOI: https://doi.org/10.1007/s11910-020-01063-1