Abstract
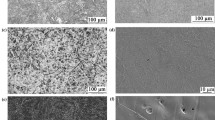
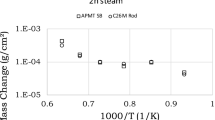
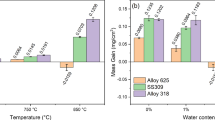
Fe and Ni base alloys serve as materials of choice for the fabrication of stack and balance of plant components in solid oxide fuel cell power systems. The oxidation behavior of select Fe and Ni base alloys has been investigated, and chromium evaporation rates have been experimentally measured at 850°C in 3% humidified air exposure conditions. Changes in the oxide morphology and chemistry from the alloys representing chromia former (602CA), alumina former (H214), and mixed chromia and alumina scale formers (AFA25) at 900°C and 1100°C during exposure to humidified air have been studied and are presented. It is found that the rate of Cr evaporation from the alloys decreases in the order of 602CA followed by Haynes 214 and AFA25 at 850°C during exposure to humidified air. Surface and cross-sectional scale morphologies and chemistry have been examined, and variations in the Cr evaporation behavior of different alloys are discussed based on the reaction thermodynamics.








Similar content being viewed by others
References
H. Yokokawa, T. Horita, N. Sakai, K. Yamaji, M. Brito, Y. Xiong, and H. Kishimoto, Solid State Ion. 177, 3193 (2006).
K. Hilpert, J. Electrochem. Soc. 143, 3642 (1996).
C. Collins, J. Lucas, T.L. Buchanan, M. Kopczyk, A. Kayani, P.E. Gannon, M.C. Deibert, and R.J. Smith, et al., Surf. Coat. Technol. 201, 4467 (2006).
J.W. Fergus, Int. J. Hydrogen Energy 32, 3664 (2007).
S.P. Jiang and X. Chen, Int. J. Hydrogen Energy 39, 505 (2014).
J. Huang, F. Xie, C. Wang, and Z. Mao, Int. J. Hydrogen Energy 37, 877 (2012).
T. Horita, Y. Xiong, H. Kishimoto, K. Yamaji, M.E. Brito, and H. Yokokawa, J. Electrochem. Soc. 157, B614 (2010).
B. Hu, S. Krishnan, C. Liang, S.J. Heo, A.N. Aphale, R. Ramprasad, and P. Singh, Int. J. Hydrogen Energy 42, 10208 (2017).
S. Taniguchi, M. Kadowaki, H. Kawamura, T. Yasuo, Y. Akiyama, Y. Miyake, and T. Saitoh, J. Power Sour. 55, 73 (1995).
E.J. Opila, D.L. Myers, N.S. Jacobson, I.M.B. Nielsen, D.F. Johnson, J.K. Olminsky, and M.D. Allendorf, J. Phys. Chem. A 111, 1971 (2007).
Y.L. Liu, A. Hagen, R. Barfod, M. Chen, H.J. Wang, F.W. Poulsen, and P.V. Hendriksen, Solid State Ion. 180, 1298 (2009).
X. Chen, P.Y. Hou, C.P. Jacobson, S.J. Visco, and L.C. De Jonghe, Solid State Ion. 176, 425 (2005).
P. Singh and N. Birks, Oxid. Met. 12, 23 (1978).
S.R. Akanda, M.E. Walter, N.J. Kidner, and M.M. Seabaugh, Thin Solid Films 565, 237 (2014).
H. 214 Brochure, http://haynesintl.com/docs/default-source/pdfs/new-alloy-brochures/high-temperature-alloys/brochures/214-brochure.pdf?sfvrsn=10.
V.P. Deodeshmukh and S.K. Srivastava, Superalloys 2008, in 11th International Symposium on Superalloys, p. 689 (2008).
R. Pillai, H. Ackermann, H. Hattendorf, and S. Richter, Corros. Sci. 75, 28 (2013).
A. Chyrkin, R. Pillai, T. Galiullin, E. Wessel, D. Druner, and W.J. Quadakkers, Corros. Sci. 127, 27 (2017).
M.P. Brady, Y. Yamamoto, M.L. Santella, and L.R. Walker, Oxid. Met. 72, 311 (2009).
Acknowledgements
Authors acknowledge the financial support from the US DOE (DE-FE-0023385). The University of Connecticut is acknowledged for providing instruments and laboratory facilities for the timely execution of the experimental work. The authors also acknowledge Dr. Lichun Zhang for his assistance in conducting FIB/TEM analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aphale, A.N., Hu, B., Reisert, M. et al. Oxidation Behavior and Chromium Evaporation From Fe and Ni Base Alloys Under SOFC Systems Operation Conditions. JOM 71, 116–123 (2019). https://doi.org/10.1007/s11837-018-3188-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-018-3188-2