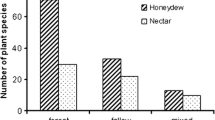
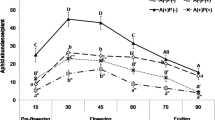
Abstract
The two most important ant–plant attractions are extrafloral nectaries (EFNs) and hemipteran honeydew. In both cases, ants may offer an effective protection against natural enemies of plants, in exchange for its sugar-rich exudates. The aim of this paper was to compare the efficiency of ant protection between plants with EFNs and with hemipteran honeydew. The study was carried out in the Amazonian Rain Forest Reserve at km 41 (02º 24′S, 59º 44′W), located 80 km from Manaus. We recorded 24 ant species in 25 plants species in the forest understory along two line transects of 5 km. The efficiency of ants in protecting plants was measured by an experiment of prey removal using isopteran workers. It was found that ants are more effective and faster in attacking termites when using honeydew rather than EFNs, probably due to the larger resource monopolization. This study further underlines the importance of experimental studies to elucidate the ecological and evolutionary importance of EFNs and honeydew in ant–plant defense against herbivores.

Similar content being viewed by others
References
Beattie AI (1985) Evolutionary ecology of ant-plant mutualisms. Cambridge University Press, Cambridge Massachusetts
Becerra JX, Venable DL (1989) Extrafloral nectaries: a defence against ant-homoptera mutualism? Oikos 55:276–280
Becerra JX, Venable DL (1991) The role of ant-homoptera mutualisms in the evolution of extrafloral nectaries. Oikos 60:105–106
Bentley BL (1977) The protective function of ants visiting extrafloral nectarines of Bixa orellanna L. (Bixacea). J Ecol 65:27–38
Blüthgen N, Verhaagh M, Goitia W, Jaffe K, Morawetz W, Barthlott W (2000) How plants shape the ant community in the Amazonian rainforest canopy: the key role of extrafloral nectaries and homopteran honeydew. Oecologia 125:229–240
Blüthgen N, Stork NE, Fiedler K (2004) Bottom-up control and co-occurrence in complex communities: honeydew and nectar determine a rainforest ant mosaic. Oikos 106:344–358
Buckley RC (1987) Ant-plant-homopteran interactions. Adv Ecol Res 16:53–85
Carver M, Gross GF, Woodward TE (1991) The insects of Australia. Cornell University Press, Ithaca
Crawley MJ (2013) The R book. Wiley, Hoboken
Cushman JH, Beattie AI (1991) Mutualisms: assessing the benefits to hosts and visitors. Trends Ecol Evol 6:193–195
Cushman JH, Whitham TG (1991) Competition mediating the outcome of a mutualism—protective services of ants as a limiting resource for membracids. Am Nat 138:851–865
Dattilo W, Marquitti FMD, Guimarães PR Jr, Izzo TJ (2014) The structure of ant-plant ecological networks: is abundance enough? Ecology 95:475–485
Dáttilo W, Guimarães PR, Izzo TJ (2013) Spatial structure of ant-plant mutualistic networks. Oikos 122:1643–1648
Davidson DW, McKey D (1993) The evolutionary ecology of symbiotic ant-plant relationships. J Hym Res 2:13–83
Delabie JHC (2001) Trophobiosis between Formicidae and Hemiptera (Sternorrhyncha and Auchenorrhyncha): an overview. Neotrop Entomol 30:501–516
Del-Claro K, Santos JC (2000) A função de nectários extraflorais em plantas do cerrado. In: Cavalcanti TB (ed) Tópicos atuais em botânica. Embrapa, Brasília, pp 84–89
Eisner T (1957) A comparative morphological study of the proventriculus of ants (Hymenoptera: Formicidae). Bull Mus Comp Zool 116:441–490
Erwin TL (1983) Tropical forest canopies: the last biotic frontier. Bull Ent Soc Amer 29:14–19
Fiala B (1990) Extrafloral nectaries vs ant-homoptera mutualisms: a comment on Becerra and Venable. Oikos 59:281–282
Floren A, Linsenmair KL (2000) Do ant mosaics exist in pristini lowland rain forests? Oecologia 123:129–137
Gaume L, Mckey D, Terrin S (1998) Ant-plant-homopteran mutualism: how the third partner affects the interaction between a plant-specialist ant and its myrmecophyte host. Proc R Soc Lond 265:567–575
Hölldobler B, Wilson EO (1990) The ants. The belknap press of harvard university press, Cambridge Massachusetts
Jeanne RL (1979) A latitudinal gradient in rates of ant predation. Ecology 60:1211–1224
Katayama N, Suzuky N (2003) Changes in the use of extrafloral nectaries of Vicia faba (Leguminosae) and honeydew of aphids by ants with increasing aphid density. Ann Entomol Soc Am 96:579–584
Korndorfer AP, Del-Claro K (2006) Ant defense versus induced defense in Lafoensia pacari (Lythraceae), a myrmecophilous tree of the Brazilian Cerrado. Biotropica 38:786–788
Moya-Raygoza G, Larsen KJ (2001) Temporal resource switching by ants between honeydew produced by the fivespotted gama grass leafhopper (Dalbulus quinquenotatus) and nectar produced by plants with extrafloral nectaries. Am Midl Nat 146:311–320
Moya-Raygoza G, Nault LR (2000) Obligatory mutualism between Dalbulus quinquenotatus (Homoptera: Cicadellidae) and attendant ants. Ann Entomol Soc Am 93:929–940
Nascimento EA, Del-Claro K (2010) Ant visitation to extrafloral nectaries decreases herbivory and increases fruit set in Chamaecrista debilis (Fabaceae) in a Neotropical savanna. Flora 205:754–756
Nogueira A, Guimarães E, Machado SR, Lohmann LG (2012) Do extrafloral nectaries present a defensive role against herbivores in two species of the family Bignoniaceae in a Neotropical savannas? Plant Ecol 213:289–301
Offenberg J (2000) Correlated evolution of the association between aphids and ants and the association between aphids and plants with extrafloral nectaries. Oikos 91:146–152
Oliveira PS, Brandão CRF (1991) The ant community associated with extrafloral nectaries in the Brazilian cerrados. In: Cutler DF, Huxley CR (eds) Ant-plant interactions. Oxford University, England, pp 198–212
Oliveira PS, Pie MR (1998) Interaction between ants and plants bearing extrafloral nectaries in cerrado vegetation. An Soc Entomol Bras 27:161–176
Oliveira OS, da Silva AF, Martins AB (1987a) Ant foraging on extrafloral nectaries of Qualea grandiflora (Vochysiaceae) in Cerrado vegetation: ants as potential anti-herbivore Agents. Oecologia 74:228–230
Oliveira PS, Oliveira-Filho AR, Cintra R (1987b) Ant-foraging on ant-inhabited Triplaris (Polygonaceae) in western Brazil: a field experiment using live termite baits. J Trop Ecol 3:195–200
R Development Core Team (2009) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rico-Gray V, Oliveira PS (2007) The Ecology and evolution of ant-plant interactions. The University of Chicago Press, Chicago
Shenoy M, Radhika V, Satish S, Borges RM (2012) Composition of extrafloral nectar influences interactions between the myrmecophyte Humboldtia brunonis and its ant associates. J Chem Ecol 38:88–99
Weibull WA (1951) A statistical distribution of wide applicability. J Appl Mech 18:293–297
Zar JH (1999) Biostatistical analysis. Prentice-Hall, Englewood Cliffs
Acknowledgments
We would like to thank the coordinators of the Amazonian Forest Ecology field course Prof. Dr. Jansen Zuanon, Prof. Dr. Eduardo Venticinque and Prof. Dr. Glauco Machado. We are grateful to Prof. Marcelo Moreira for the plant identification, to Flávio Quental for help in the field and to Angela Pacheco for help in data analyses. We are also grateful to Prof. Dr. Heraldo Luís de Vasconcelos, Andrew White and two anonymous referees for manuscript revision.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editors: Stanislav Gorb and Heikki Hokkanen.
Rights and permissions
About this article
Cite this article
Campos, R.I., Camacho, G.P. Ant–plant interactions: the importance of extrafloral nectaries versus hemipteran honeydew on plant defense against herbivores. Arthropod-Plant Interactions 8, 507–512 (2014). https://doi.org/10.1007/s11829-014-9338-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-014-9338-8