Abstract
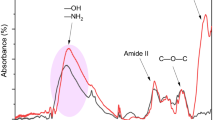
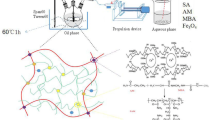
Aminated cassava residue magnetic microspheres (ACRPM) were synthesized via an inverse emulsion method by using chemically modified cassava residue as a crude material, and acrylic acid (AA), acrylamide (AM), and methyl methacrylate (MMA) as monomers and a polyethylene glycol/methanol system (PEG/MeOH) as the porogen. Fourier-transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), N2 adsorption-desorption and vibrating sample magnetometry (VSM) were used to characterize the ACRPM. The results indicated that amino groups were grafted to the cassava residue magnetic microspheres, and the Fe3O4 nanoparticles were encapsulated in the microspheres. After porogen was added, the particle size of the ACRPM decreased from 16.5 μm to 150 nm with a pore volume of 0.05510 m3/g, and the specific surface area of the ACRPM increased from 3.02 to 12.34 m2/g. The ACRPM were superparamagnetic, and the saturation magnetization was 9.8 emu/g. The maximum adsorption capacity of Pb(II) on the ACRPM was 390 mg/g. The ACRPM exhibited a large specific surface area and provided many adsorption sites for metal ion adsorption, which favored a high adsorption capacity. Additionally, the Pb(II) adsorption process was fitted to pseudo-second-order kinetic and Langmuir isothermal adsorption models. This suggests that the Pb(II) adsorption process was dominated by a chemical reaction process and that chemisorption was the rate-controlling step during the Pb(II) removal process. In addition, the adsorbent exhibited good stability after six consecutive reuses.
Similar content being viewed by others
References
E. Da’na, Micropor. Mesopor. Mater., 247, 145 (2017).
G. Tepanosyan, L. Sahakyan, O. Belyaeva, N. Maghakyan and A. Saghatelyan, Chemosphere, 184, 1230 (2017).
J. Ma, Y. Liu, O. Ali, Y. Wei, S. Zhang, Y. Zhang, T. Cai, C. Liu and S. Luo, J. Hazard. Mater., 344, 1034 (2018).
T. A. Kurniawan, G.Y. Chan, W. H. Lo and S. Babel, Sci. Total Environ., 366, 409 (2006).
Y. Ma, L. Lv, Y. Guo, Y. Fu, Q. Shao, T. Wu, S. Guo, K. Sun, X. Guo, E.K. Wujcik and Z. Guo, Poly, 128, 12 (2017).
J. Huang, Y. Cao, Q. Shao, X. Peng and Z. Guo, Ind. Eng. Chem. Res., 56, 10689 (2017).
U.K. Garg, M. P. Kaur, V. K. Garg and D. Sud, J. Hazard. Mater., 140, 60 (2007).
M. I. Shariful, T. Sepehr, M. Mehrali, B.C. Ang and M.A. Amalina, J. Appl. Polym. Sci., 135, 45851 (2018).
Z. Xu, G. Gao, B. Pan, W. Zhang and L. Lv, Water Res., 87, 378 (2015).
I. Petrinic, J. Korenak, D. Povodnik and C. Hélix-Nielsen, J. Clean. Prod., 101, 292 (2015).
J. Altmann, A. S. Ruhl, F. Zietzschmann and M. Jekel, Water Res., 55, 185 (2014).
M.A. Shaker and H. M. albishri, Chemosphere, 111, 587 (2014).
N. Li, F. Fu, J. Lu, Z. Ding, B. Tang and J. Pang, Environ. Pollut., 220, 1376 (2017).
L. Lv, N. Chen, C. Feng, J. Zhang and M. Li, RSC Adv., 7, 27992 (2017).
H.-C. Dang, X. Yuan, Q. Xiao, W.-X. Xiao, Y.-K. Luo, X.-L. Wang, F. Song and Y.-Z. Wang, J. Environ. Chem. Eng., 5, 4505 (2017).
N. M. Noor, R. Othman, N.M. Mubarak and E. C. Abdullah, J. Taiwan Inst. Chem. Eng., 78, 168 (2017).
W. Park, A.C. Gordon, S. Cho, X. Huang, K.R. Harris, A.C. Larson and D.-H. Kim, ACS Appl. Mater. Inter., 9, 13819 (2017).
N. Rodkate and M. Rutnakornpituk, Carbohyd. Polym., 151, 251 (2016).
X. Zhang, N. Zhang, C. Du, P. Guan, X. Gao, C. Wang, Y. Du, S. Ding and X. Hu, Chem. Eng. J., 317, 988 (2017).
Z. Hu, Q. Shao, M. G. Moloney, X. Xu, D. Zhang, J. Li, C. Zhang and Y. Huang, Macromolecules, 50, 1422 (2017).
W. Zhu, W. Ma, C. Li, J. Pan and X. Dai, Chem. Eng. J., 276, 249 (2015).
J. Liu, H.-T. Wu, J.-f. Lu, X.-y. Wen, J. Kan and C.-h. Jin, Chem. Eng. J., 262, 803 (2015).
J. Xie, G. Zhong, C. Cai, C. Chen and X. Chen, Talanta, 169, 98 (2017).
J. Huang, P. Su, L. Zhou and Y. Yang, Colloids Surf., A, 490, 241 (2016).
P. Pingmuanglek, N. Jakrawatana and S. H. Gheewala, J. Clean. Prod., 162, 1075 (2017).
H. Jiang, Y. Qin, S. I. Gadow and Y.-Y. Li, Int. J. Hydrogen Energy, 42, 2868 (2017).
H. Lu, C. Lv, M. Zhang, S. Liu, J. Liu and F. Lian, Energy Convers. Manage., 132, 251 (2017).
J. Cheng, J. Zhang, R. Lin, J. Liu, L. Zhang and K. Cen, Bioresour. Technol., 228, 348 (2017).
X. Xie, H. Xiong, Y. Zhang, Z. Tong, A. Liao and Z. Qin, J. Environ. Chem. Eng., 5, 2800 (2017).
A.R. Garcia, C. Lacko, C. Snyder, A. C. Bohórquez, C. E. Schmidt and C. Rinaldi, Colloids Surf. Physicochem. Eng. Aspects, 529, 119 (2017).
Z. Guo, J. Fan, J. Zhang, Y. Kang, H. Liu, L. Jiang and C. Zhang, J. Taiwan Inst. Chem. Eng., 58, 290 (2016).
A. Hajlane, H. Kaddami and R. Joffe, Ind. Crop. Prod., 100, 41 (2017).
D. Morillo Martín, M. Faccini, M.A. García and D. Amantia, J. Environ. Chem. Eng., 6, 236 (2018).
Q. Lin, J. Pan, Q. Lin and Q. Liu, J. Hazard. Mater., 263, 517 (2013).
L. Lu, J. Li, D. H. L. Ng, P. Yang, P. Song and M. Zuo, J. Ind. Eng. Chem., 46, 315 (2017).
W. Wang, T. Liang, H. Bai, W. Dong and X. Liu, Carbohydr. Polym., 179, 297 (2018).
T. Zhai, Q. Zheng, Z. Cai, H. Xia and S. Gong, Carbohydr. Polym., 148, 300 (2016).
L. Wang and D.E. Giammar, J. Colloid Interface Sci., 448, 331 (2015).
X. Liu, M. Liu and L. Zhang, J. Colloid Interface Sci., 511, 135 (2018).
D. Kolodynska, J. Krukowska-Bak, J. Kazmierczak-Razna and R. Pietrzak, Micropor. Mesopor. Mater., 244, 127 (2017).
N. A. Fakhre and B.M. Ibrahim, J. Hazard. Mater., 343, 324 (2018).
X. Ma, X. Liu, D. P. Anderson and P.R. Chang, Food Chem., 181, 133 (2015).
Q. Liu, F. Li, H. Lu, M. Li, J. Liu, S. Zhang, Q. Sun and L. Xiong, Food Chem., 242, 256 (2018).
N. Yin, K. Wang, Y. A. Xia and Z. Li, Desalination, 430, 120 (2018).
T. Liu, X. Han, Y. Wang, L. Yan, B. Du, Q. Wei and D. Wei, J. Colloid Interface Sci., 508, 405 (2017).
Q. Yuan, Y. Chi, N. Yu, Y. Zhao, W. Yan, X. Li and B. Dong, Mater. Res. Bull., 49, 279 (2014).
H. L. Fan, S. F. Zhou, W. Z. Jiao, G. S. Qi and Y. Z. Liu, Carbohyd. Polym., 174, 1192 (2017).
Q. Hu, Z. Xiao, X. Xiong, G. Zhou and X. Guan, J. Environ. Sci., 27, 207 (2015).
J.N. Putro, S. P. Santoso, S. Ismadji and Y.-H. Ju, Micropor. Mesopor. Mater., 246, 166 (2017).
A.A. Yakout, R. H. El-Sokkary, M. A. Shreadah and O. G. Abdel Hamid, Carbohyd. Polym., 172, 20 (2017).
T.W. Cheng, M. L. Lee, M. S. Ko, T. H. Ueng and S. F. Yang, Appl. Clay Sci., 56, 90 (2012).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Xie, X., Huang, J., Zhang, Y. et al. Aminated cassava residue-based magnetic microspheres for Pb(II) adsorption from wastewater. Korean J. Chem. Eng. 36, 226–235 (2019). https://doi.org/10.1007/s11814-018-0190-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-018-0190-x