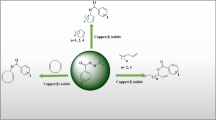
Abstract
Reductive iodonio-Claisen rearrangement (RICR) involving λ3-iodanes and allyl or substituted-allyl silanes in fluoroalcohols, such as 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) and 2,2,2-trifluoroethanol (TFE), was studied for the synthesis of complex ortho-allyl or substituted-allyl iodoarenes. In comparison to the previously reported condition involving boron trifluoride diethyl etherate, the RICR mediated by fluoroalcohols was found to proceed more effectively. The resulting complex ortho-allyl iodoarenes are useful synthetic intermediates and can be readily converted to various heterocyclic compounds.

Similar content being viewed by others
References
Stang P J, Zhdankin V V. Organic polyvalent iodine compounds. Chemical Reviews. 1996, 96(3): 1123–1178
Zhdankin V V, Stang P J. Recent developments in the chemistry of polyvalent iodine compounds. Chemical Reviews. 2002, 102(7): 2523–2584
Zhdankin V V, Stang P J. Chemistry of polyvalent iodine. Chemical Reviews. 2008, 108(12): 5299–5358
Ochiai M. Hypervalent halogans and their reactions in organic synthesis. Yakugaku Zasshi. Journal of the Pharmaceutical Society of Japan. 2009, 129(3): 321–334
Zhdankin V V. Hypervalent iodine (III) reagents in organic synthesis. ARKIVOC. 2009, 1(1): 1–62
Merritt E A, Olofsson B. α-Functionalization of carbonyl compounds using hypervalent iodine reagents. Synthesis (Stuttgart), 2011, 4: 517–538
Silva L F Jr, Olofsson B. Hypervalent iodine reagents in the total synthesis of natural products. Natural Product Reports. 2011, 28(10): 1722–1754
Duschek A, Kirsch S F. 2-Iodoxybenzoic acid—a simple oxidant with a dazzling array of potential applications. Angewandte Chemie International Edition, 2011, 50(7): 1524–1552
Duschek A, Kirsch S. For an overview of IBX-mediated oxidations. Angewandte Chemie. 2011, 123: 1562–1590
Ochiai M, Ito T, Takaoka Y, Masaki Y. Generation of allenyliodinanes and their reductive iodonio-Claisen rearrangement. Journal of the American Chemical Society. 1991, 113(4): 1319–1323
Ochiai M, Ito T, Masaki Y. Ipso selectivity in the reductive iodonio-Claisen rearrangement of allenyl(p-methoxyaryl)iodinanes. Journal of the Chemical Society. Chemical Communications. 1992, (1): 15–16
Ochiai M, Ito T. Solvent effects on ipso versus ortho selectivity in the reductive iodonio-Claisen rearrangement of allenyl (p-methoxyphenyl) iodane. Journal of Organic Chemistry. 1995, 60(7): 2274–2275
Ochiai M, Kida M, Okuyama T. On the mechanism of nucleophilic substitution of allenyl (aryl) iodine (III): Formation of propargyl cation and competition with Sigmatropic rearrangement. Tetrahedron Letters. 1998, 39(34): 6207–6210
Gately D A, Luther T A, Norton J R, Miller M M, Anderson O P. Reaction of µ-oxobis [(trifluoromethanesulfonato)(phenyl) iodine (III)] with group 14 propargyl derivatives and a propargyl ether. Journal of Organic Chemistry. 1992, 57(24): 6496–6502
Lee K, Kim D Y, Oh D Y. Reaction of allyltrimethylsilane with an aromatic compound using hypervalent organoiodine compound: A new allylation of aromatic compounds. Tetrahedron Letters. 1988, 29(6): 667–668
Van De Water R W, Hoarau C, Pettus T R R. Oxidative dearomatization of resorcinol derivatives: Useful conditions leading to valuable cyclohexa-2,5-dienones. Tetrahedron Letters. 2003, 44(27): 5109–5113
Zhu J, Germain A R, Porco J A. Synthesis of azaphilones and related molecules by employing cycloisomerization of O-alkynylbenzaldehydes. Angewandte Chemie International. 2004, 43(10): 1239–1243
Jia Z, Galvez E, Sebastian R M, Pleixats R, Alvarez-Larena A, Martin E, Vallribera A, Shafir A. An alternative to the classical alpha-arylation: The transfer of an intact 2-iodoaryl from ArI (O2CCF3)2. Angewandte Chemie International Edition. 2014, 53(42): 11298–11301
Khatri H R, Zhu J. Synthesis of complex ortho-allyliodoarenes employing reductive iodonio-Claisen rearrangement. Chemistry (Weinheim an der Bergstrasse, Germany). 2012, 18(39): 12232–12236
Nguyen H, Khatri H R, Zhu J. Reductive iodonio-Claisen rearrangement of iodothiophene diacetates with allylsilanes: Formal synthesis of Plavix®. Tetrahedron Letters. 2013, 54(40): 5464–5466
Bonnet-Delpon D, Bégué J P, Crousse B. Fluorinated alcohols: A new medium for selective and clean reaction. Synlett. 2004, (1): 18–29
Dohi T, Yamaoka N, Kita Y. Fluoroalcohols: Versatile solvents in hypervalent iodine chemistry and syntheses of diaryliodonium(III) salts. Tetrahedron. 2010, 66(31): 5775–5785
Kita Y, Yakura T, Tohma H, Kikuchi K, Tamura Y. A synthetic approach to discorhabdin alkaloids: Hypervalent iodine oxidation of p-substituted phenol derivatives to azacarbocyclic spirodienones. Tetrahedron Letters. 1989, 30(9): 1119–1120
Kita Y, Tohma H, Kikuchi K, Inagaki M, Yakura T. Hypervalent iodine oxidation of N-acyltyramines: Synthesis of quinol ethers, spirohexadienones, and hexahydroindol-6-ones. Journal of Organic Chemistry. 1991, 56(1): 435–438
Kita Y, Tohma H, Inagaki M, Hatanaka K, Kikuchi K, Yakura T. Hypervalent iodine oxidation of O-silylated phenol derivatives to azacarbocyclic spirodienones; Synthetic approach to the anticancer marine alkaloid, Discorhabdin C. Tetrahedron Letters. 1991, 32(18): 2035–2038
Kita Y, Tohma H, Inagaki M, Hatanaka K, Yakura T. Total synthesis of discorhabdin C: A general aza spiro dienone formation from O-silylated phenol derivatives using a hypervalent iodine reagent. Journal of the American Chemical Society. 1992, 114(6): 2175–2180
Kita Y, Arisawa M, Gyoten M, Nakajima M, Hamada R, Tohma H, Takada T. Oxidative intramolecular phenolic coupling reaction induced by a hypervalent iodine (III) reagent: Leading to galanthamine-type amaryllidaceae alkaloids. Journal of Organic Chemistry. 1998, 63(19): 6625–6633
Tohma H, Harayama Y, Hashizume M, Iwata M, Kiyono Y, Egi M, Kita Y. The first total synthesis of Discorhabdin A. Journal of the American Chemical Society. 2003, 125(37): 11235–11240
Kita Y, Tohma H, Hatanaka K, Takada T, Fujita S, Mitoh S, Sakurai H, Oka S. Hypervalent iodine-induced nucleophilic substitution of para-substituted phenol ethers. Generation of cation radicals as reactive intermediates. Journal of the American Chemical Society. 1994, 116(9): 3684–3691
Kita Y, Takada T, Mihara S, Whelan B A, Tohma H. Novel and direct nucleophilic sulfenylation and thiocyanation of phenol ethers using a hypervalent iodine (III) reagent. Journal of Organic Chemistry. 1995, 60(22): 7144–7148
Dohi T, Ito M, Morimoto K, Minamitsuji Y, Takenaga N, Kita Y. Versatile direct dehydrative approach for diaryliodonium (III) salts in fluoroalcohol media. Chemical Communications. 2007, 40(40): 4152–4154
Schadt F L, Bentley T W, Schleyer P R. The SN2-SN1 spectrum. 2. Quantitative treatments of nucleophilic solvent assistance. A Scale of solvent nucleophilicities. Journal of the American Chemical Society, 1976, 98(24): 7667–7675
Kuwabe S, Torraca K E, Buchwald S L. Palladium-catalyzed intramolecular C–O bond formation. Journal of the American Chemical Society. 2001, 123(49): 12202–12206
Shafir A, Buchwald S L. Highly selective room-temperature coppercatalyzed C–N coupling reactions. Journal of the American Chemical Society. 2006, 128(27): 8742–8743
Che C Y, Dormer P G. Synthesis of benzo [b] furans via CuIcatalyzed ring closure. Journal of Organic Chemistry. 2005, 70(17): 6964–6967
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the 120th Anniversary of Tianjin University
Rights and permissions
About this article
Cite this article
Khatri, H.R., Nguyen, H., Dunaway, J.K. et al. Fluoroalcohol-mediated reductive iodonio-Claisen rearrangement: Synthesis of complex ortho-substituted-allyl iodoarenes. Front. Chem. Sci. Eng. 9, 359–368 (2015). https://doi.org/10.1007/s11705-015-1530-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-015-1530-6