Abstract
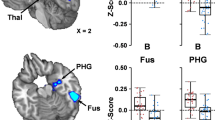
The goal of this pilot study is to use complementary MRI strategies to quantify and relate cerebrovascular reactivity, resting cerebral blood flow and functional connectivity alterations in the first week following sports concussion in college varsity athletes. Seven college athletes (3F/4M, age = 19.7 ± 1.2 years) were imaged 3–6 days following a diagnosed sports related concussion and compared to eleven healthy controls with no history of concussion (5M/6F, 18–23 years, 7 athletes). Cerebrovascular reactivity and functional connectivity were measured using functional MRI during a hypercapnia challenge and via resting-state regional partial correlations, respectively. Resting cerebral blood flow was quantified using arterial spin labeling MRI methods. Group comparisons were made within and between 18 regions of interest. Cerebrovascular reactivity was increased after concussion when averaged across all regions of interest (p = 0.04), and within some default-mode network regions, the anterior cingulate and the right thalamus (p < 0.05) independently. The FC was increased in the concussed athletes within the default-mode network including the left and right hippocampus, precuneus and ventromedial prefrontal cortex (p < 0.01), with measures being linearly related to cerebrovascular reactivity in the hippocampus in the concussed athletes. Significant resting cerebral blood flow changes were not detected between the two groups. This study provides evidence for increased cerebrovascular reactivity and functional connectivity in the medial regions of the default-mode network within days of a single sports related concussion in college athletes. Our findings emphasize the utility of complementary cerebrovascular measures in the interpretation of alterations in functional connectivity following concussion.




Similar content being viewed by others
References
Abbas, K., Shenk, T. E., Poole, V. N., Breedlove, E. L., Leverenz, L. J., Nauman, E. A., et al. (2014). Alteration of default mode network in high school football athletes due to repetitive subconcussive mild traumatic brain injury: a resting-state functional magnetic resonance imaging study. Brain Connect. doi:10.1089/brain.2014.0279.
Bartnik-Olson, B. L., Holshouser, B., Wang, H., Grube, M., Tong, K., Wong, V., et al. (2014). Impaired neurovascular unit function contributes to persistent symptoms after concussion: a pilot study. Journal of Neurotrauma, 31(17), 1497–1506. doi:10.1089/neu.2013.3213.
Becelewski, J., & Pierzchala, K. (2003). Cerebrovascular reactivity in patients with mild head injury. Neurologia i Neurochirurgia Polska, 37(2), 339–350.
Biswal, B., Yetkin, F. Z., Haughton, V. M., & Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine, 34(4), 537–541.
Bonne, O., Gilboa, A., Louzoun, Y., Kempf-Sherf, O., Katz, M., Fishman, Y., et al. (2003). Cerebral blood flow in chronic symptomatic mild traumatic brain injury. Psychiatry Research: Neuroimaging, 124(3), 141–152. doi:10.1016/s0925-4927(03)00109-4.
Bright, M. G., & Murphy, K. (2013). Reliable quantification of BOLD fMRI cerebrovascular reactivity despite poor breath-hold performance. NeuroImage, 83, 559–568. doi:10.1016/j.neuroimage.2013.07.007.
Buckner, R. L., Andrews-Hanna, J. R., & Schacter, D. L. (2008). The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124, 1–38.
Buxton, R. B., Frank, L. R., Wong, E. C., Siewert, B., Warach, S., & Edelman, R. R. (1998). A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magnetic Resonance in Medicine, 40(3), 383–396. doi:10.1002/mrm.1910400308.
Chen, Y. F., Wang, D. J. J., & Detre, J. A. (2011). Test-retest reliability of arterial spin labeling with common labeling strategies. Journal of Magnetic Resonance Imaging, 33(4), 940–949. doi:10.1002/jmri.22345.
Clark, R. S. B., Kochanek, P. M., Schwarz, M. A., Schiding, J. K., Turner, D. S., Chen, M. Z., et al. (1996). Inducible nitric oxide synthase expression in cerebrovascular smooth muscle and neutrophils after traumatic brain injury in immature rats. Pediatric Research, 39(5), 784–790. doi:10.1203/00006450-199605000-00007.
Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale: Lawrence Earlbaum Associates.
Cordes, D., Haughton, V. M., Arfanakis, K., Carew, J. D., Turski, P. A., Moritz, C. H., et al. (2001). Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. American Journal of Neuroradiology, 22(7), 1326–1333.
Dai, W. Y., Garcia, D., de Bazelaire, C., & Alsop, D. C. (2008). Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magnetic Resonance in Medicine, 60(6), 1488–1497. doi:10.1002/mrm.21790.
Detre, J. A., Leigh, J. S., Williams, D. S., & Koretsky, A. P. (1992). Perfusion imaging. Magnetic Resonance in Medicine, 23(1), 37–45. doi:10.1002/mrm.1910230106.
Gahm, C., Holmin, S., & Mathiesen, T. (2000). Temporal profiles and cellular sources of three nitric oxide synthase isoforms in the brain after experimental contusion. Neurosurgery, 46(1), 169–177.
Gao, W., & Lin, W. L. (2012). Frontal parietal control network regulates the anti-correlated default and dorsal attention networks. Human Brain Mapping, 33(1), 192–202. doi:10.1002/hbm.21204.
Ge, Y. L., Patel, M. B., Chen, Q., Grossman, E. J., Zhang, K., Miles, L., et al. (2009). Assessment of thalamic perfusion in patients with mild traumatic brain injury by true FISP arterial spin labelling MR imaging at 3T. Brain Injury, 23(7–8), 666–674. doi:10.1080/02699050903014899.
Glover, G. H., Li, T. Q., & Ress, D. (2000). Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magnetic Resonance in Medicine, 44(1), 162–167.
Grindel, S. H., Lovell, M. R., & Collins, M. W. (2001). The assessment of sport-related concussion: the evidence behind neuropsychological testing and management. Clinical Journal of Sport Medicine, 11(3), 134–143. doi:10.1097/00042752-200107000-00003.
Hare, H. V., Germuska, M., Kelly, M. E., & Bulte, D. P. (2013). Comparison of CO2 in air versus carbogen for the measurement of cerebrovascular reactivity with magnetic resonance imaging. Journal of Cerebral Blood Flow and Metabolism, 33(11), 1799–1805. doi:10.1038/jcbfm.2013.131.
Jiang, L., Kim, M., Chodkowski, B., Donahue, M. J., Pekar, J. J., Van Zijl, P. C. M., et al. (2010). Reliability and reproducibility of perfusion MRI in cognitively normal subjects. Magnetic Resonance Imaging, 28(9), 1283–1289. doi:10.1016/j.mri.2010.05.002.
Johnson, B., Zhang, K., Gay, M., Horovitz, S., Hallett, M., Sebastianelli, W., et al. (2012). Alteration of brain default network in subacute phase of injury in concussed individuals: resting-state fMRI study. NeuroImage, 59(1), 511–518. doi:10.1016/j.neuroimage.2011.07.081.
Johnson, B., Neuberger, T., Gay, M., Hallett, M., & Slobounov, S. (2014). Effects of subconcussive head trauma on the default mode network of the brain. Journal of Neurotrauma, 31(23), 1907–1913. doi:10.1089/neu.2014.3415.
Junger, E. C., Newell, D. W., Grant, G. A., Avellino, A. M., Ghatan, S., Douville, C. M., et al. (1997). Cerebral autoregulation following minor head injury. Journal of Neurosurgery, 86(3), 425–432. doi:10.3171/jns.1997.86.3.0425.
King, N. S., Crawford, S., Wenden, F. J., Moss, N. E. G., & Wade, D. T. (1995). The rivermead post concussion symptoms questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. Journal of Neurology, 242(9), 587–592. doi:10.1007/BF00868811.
Len, T. K., Neary, J. P., Asmundson, G. J., Goodman, D. G., Bjornson, B., & Bhambhani, Y. N. (2011). Cerebrovascular reactivity impairment after sport-induced concussion. Medicine and Science in Sports and Exercise, 43(12), 2241–2248. doi:10.1249/MSS.0b013e3182249539.
Len, T. K., Neary, J. P., Asmundson, G. J. G., Candow, D. G., Goodman, D. G., Bjornson, B., et al. (2013). Serial monitoring of CO2 reactivity following sport concussion using hypocapnia and hypercapnia. Brain Injury, 27(3), 346–353. doi:10.3109/02699052.2012.743185.
Liu, P. Y., Hebrank, A. C., Rodrigue, K. M., Kennedy, K. M., Section, J., Park, D. C., et al. (2013). Age-related differences in memory-encoding fMRI responses after accounting for decline in vascular reactivity. NeuroImage, 78, 415–425. doi:10.1016/j.neuroimage.2013.04.053.
Logothetis, N. K., Pauls, J., Augath, M., Trinath, T., & Oeltermann, A. (2001). Neurophysiological investigation of the basis of the fMRI signal. Nature, 412(6843), 150–157.
Lu, H. Z., Zhao, C. G., Ge, Y. L., & Lewis-Amezcua, K. (2008). Baseline blood oxygenation modulates response amplitude: Physiologic basis for intersubject variations in functional MRI signals. Magnetic Resonance in Medicine, 60(2), 364–372. doi:10.1002/mrm.21686.
Maldjian, J. A., Laurienti, P. J., Kraft, R. A., & Burdette, J. H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19(3), 1233–1239.
Maugans, T. A., Farley, C., Altaye, M., Leach, J., & Cecil, K. M. (2012). Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics, 129(1), 28–37. doi:10.1542/peds.2011-2083.
Mayer, A. R., Mannell, M. V., Ling, J., Gasparovic, C., & Yeo, R. A. (2011). Functional connectivity in mild traumatic brain injury. Human Brain Mapping, 32(11), 1825–1835. doi:10.1002/hbm.21151.
McCrory, P., Meeuwisse, W. H., Aubry, M., Cantu, B., Dvořák, J., Echemendia, R. J., et al. (2013). Consensus statement on concussion in sport: the 4th International conference on concussion in sport held in Zurich, November 2012. British Journal of Sports Medicine, 47(5), 250–258. doi:10.1136/bjsports-2013-092313.
McQuire, J. C., Sutcliffe, J. C., & Coats, T. J. (1998). Early changes in middle cerebral artery blood flow velocity after head injury. Journal of Neurosurgery, 89(4), 526–532. doi:10.3171/jns.1998.89.4.0526.
Meier, T. B., Bellgowan, P. F., Singh, R., Kuplicki, R., Polanski, D. W., & Mayer, A. R. (2015). Recovery of cerebral blood flow following sports-related concussion. JAMA Neurol. doi:10.1001/jamaneurol.2014.4778.
Murphy, K., Harris, A. D., & Wise, R. G. (2011). Robustly measuring vascular reactivity differences with breath-hold: normalising stimulus-evoked and resting state BOLD fMRI data. NeuroImage, 54(1), 369–379. doi:10.1016/j.neuroimage.2010.07.059.
Ogawa, S., Lee, T. M., Nayak, A. S., & Glynn, P. (1990). Oxygenation-sensitive contrast in magnetic-resonance image of rodent brain at high magnetic-fields. Magnetic Resonance in Medicine, 14(1), 68–78.
Ogoh, S., & Ainslie, P. N. (2009). Cerebral blood flow during exercise: mechanisms of regulation. Journal of Applied Physiology, 107(5), 1370–1380. doi:10.1152/japplphysiol.00573.2009.
Palacios, E. M., Sala-Llonch, R., Junque, C., Roig, T., Tormos, J. M., Bargallo, N., et al. (2013). Resting-state functional magnetic resonance imaging activity and connectivity and cognitive outcome in traumatic brain injury. JAMA Neurol, 70(7), 845–851. doi:10.1001/jamaneurol.2013.38.
Peebles, K. C., Richards, A. M., Celi, L., McGrattan, K., Murrell, C. J., & Ainslie, P. N. (2008). Human cerebral arteriovenous vasoactive exchange during alterations in arterial blood gases. Journal of Applied Physiology, 105(4), 1060–1068. doi:10.1152/japplphysiol.90613.2008.
Petrov, T., Page, A. B., Owen, C. R., & Rafols, J. A. (2000). Expression of the inducible nitric oxide synthase in distinct cellular types after traumatic brain injury: an in situ hybridization anal immunocytochemical study. Acta Neuropathologica, 100(2), 196–204.
Rogers, B. P., Morgan, V. L., Newton, A. T., & Gore, J. C. (2007). Assessing functional connectivity in the human brain by fMRI. Magnetic Resonance Imaging, 25(10), 1347–1357.
Sharp, D. J., Beckmann, C. F., Greenwood, R., Kinnunen, K. M., Bonnelle, V., De Boissezon, X., et al. (2011). Default mode network functional and structural connectivity after traumatic brain injury. Brain, 134, 2233–2247. doi:10.1093/brain/awr175.
Slobounov, S. M., Zhang, K., Pennell, D., Ray, W., Johnson, B., & Sebastianelli, W. (2010). Functional abnormalities in normally appearing athletes following mild traumatic brain injury: a functional MRI study. Experimental Brain Research, 202(2), 341–354. doi:10.1007/s00221-009-2141-6.
Sridharan, D., Levitin, D. J., & Menon, V. (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America, 105(34), 12569–12574. doi:10.1073/pnas.0800005105.
Strebel, S., Lam, A. M., Matta, B. F., & Newell, D. W. (1997). Impaired cerebral autoregulation after mild brain injury. Surgical Neurology, 47(2), 128–131.
Tang, L., Ge, Y. L., Sodickson, D. K., Miles, L., Zhou, Y. X., Reaume, J., et al. (2011). Thalamic resting-state functional networks: disruption in patients with mild traumatic brain injury. Radiology, 260(3), 831–840. doi:10.1148/radiol.11110014.
Tavazzi, B., Vagnozzi, R., Signoretti, S., Amorini, A. M., Belli, A., Cimatti, M., et al. (2007). Temporal window of metabolic brain vulnerability to concussions: oxidative and nitrosative stresses—part II. Neurosurgery, 61(2), 390–395. doi:10.1227/01.neu.0000255525.34956.3f.
Thomsen, L. L., Iversen, H. K., & Olesen, J. (1995). Increased cerebrovascular PCO(2) reactivity in migraine with aura—a transcranial Doppler study during hyperventilation. Cephalalgia, 15(3), 211–215. doi:10.1046/j.1468-2982.1995.015003211.x.
Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15(1), 273–289. doi:10.1006/nimg.2001.0978.
Wada, K., Chatzipanteli, K., Kraydieh, S., Busto, R., & Dietrich, W. D. (1998). Inducible nitric oxide synthase expression after traumatic brain injury and neuroprotection with aminoguanidine treatment in rats. Neurosurgery, 43(6), 1427–1436. doi:10.1097/00006123-199812000-00096.
Wang, J. J., Alsop, D. C., Song, H. K., Maldjian, J. A., Tang, K., Salvucci, A. E., et al. (2003). Arterial transit time imaging with flow encoding arterial spin tagging (FEAST). Magnetic Resonance in Medicine, 50(3), 599–607. doi:10.1002/mrm.10559.
Zhang, K., Johnson, B., Gay, M., Horovitz, S. G., Hallett, M., Sebastianelli, W., et al. (2012). Default mode network in concussed individuals in response to the YMCA physical stress test. Journal of Neurotrauma, 29(5), 756–765. doi:10.1089/neu.2011.2125.
Zhou, Y. X., Milham, M. P., Lui, Y. W., Miles, L., Reaume, J., Sodickson, D. K., et al. (2012). Default-mode network disruption in mild traumatic brain injury. Radiology, 265(3), 882–892. doi:10.1148/radiol.12120748.
Zhou, Y., Lui, Y. W., Zuo, X.-N., Milham, M. P., Reaume, J., Grossman, R. I., et al. (2013). Characterization of thalamo-cortical association using amplitude and connectivity of functional MRI in mild traumatic brain injury. Journal of Magnetic Resonance Imaging. doi:10.1002/jmri.24310.
Zhu, D. C., Covassin, T., Nogle, S., Doyle, S., Russell, D., Pearson, R. L., et al. (2014). A potential biomarker in sports-related concussion: brain functional connectivity alteration of the default-mode network measured with longitudinal resting-state fMRI over 30 days. Journal of Neurotrauma. doi:10.1089/neu.2014.3413.
Acknowledgments
This work was supported in part by Vanderbilt CTSA grant UL1 TR000445 from NCRR/NIH (Morgan).
Compliance with Ethical Standards
ᅟ
Funding
This work was supported in part by Vanderbilt CTSA grant UL1 TR000445 from NCRR/NIH (Morgan).
Conflict of interest
Adam R. Militana, Manus J. Donahue, Allen K. Sills, Gary S. Solomon, Andrew J. Gregory, Megan K. Strother, and Victoria L. Morgan declare that they have no conflict of interest.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Vanderbilt University and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all participants included in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Militana, A.R., Donahue, M.J., Sills, A.K. et al. Alterations in default-mode network connectivity may be influenced by cerebrovascular changes within 1 week of sports related concussion in college varsity athletes: a pilot study. Brain Imaging and Behavior 10, 559–568 (2016). https://doi.org/10.1007/s11682-015-9407-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-015-9407-3