Abstract
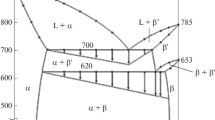
In the Au-Ba-Ge system the clathrate type I solid solution, Ba8Au x Ge46−x−y □ y , extends at 800 °C from binary Ba8Ge43□3 (□ is a vacancy) to Ba8Au6Ge40. For the clathrate phase (1 ≤ x ≤ 6) cubic primitive symmetry (space group \( Pm{\bar{{3}}}n \)) was confirmed by x-ray powder diffraction assisted by x-ray single crystal analyses of Ba8Au4.6Ge40.3□1.1. The lattice parameters of the solid solution show an almost linear increase with increasing gold content. Site preference from x-ray refinement shows that gold atoms preferably occupy the 6d site in random mixture with Ge and vacancies, which vanish at the solubility limit. Clathrate type ΙX (Ba6Ge25 type) has a maximum solubility of 2.7 at.% gold at 800 °C. Phase equlilibria at 800 °C are characterized by four ternary phases in the investigated region up to 33.3 at.% barium. The homogeneity range of Ba(Au1−x Ge x )2 (AlB2-type) and BaAu1+x Ge3−x has been established: Ba(Au1−x Ge x )2 extends from BaAu0.5Ge1.5 to BaAu0.9Ge1.1 and BaAu1+x Ge3−x from BaAu1.1Ge2.9 (BaNiSn3-type) to BaAu2.7Ge1.3 (Ce(Ni,Sb)4-type). The crystal structures of two phases in the gold-rich part have been determined from single crystal x-ray data and were found to form new structure types: BaAu3Ge with BaAu3Ge-type (space group P4/nmm, a = 0.6459(2), c = 0.5487(2) nm) and BaAu5+x Ge2−x (x = 0, BaAu5Si2-type, space group Pnma, a = 0.8981(2), b = 0.7106(2) and c = 1.0363(2) nm), the latter revealing with increasing gold content a closely related derivative structure type (BaAu5.3Ge1.7, \( a = a_{{{\text{BaAu}}_{5} {\text{Si}}_{2} }} ,\;b = b_{{{\text{BaAu}}_{5} {\text{Si}}_{2} }} ,\;c = 2c_{{{\text{BaAu}}_{5} {\text{Si}}{}_{2}}} \)). Transport properties and particularly the thermoelectric behavior were studied for Ba8Au6Ge40.













Similar content being viewed by others
Notes
During preparation of these manuscript, we learned to know a Ref 23 reporting a split position (25% occupation of Ba2 in an off center position at a 24k site) in Ba8Au5.3Ge40.7. However our Difference Fourier map and SC refinement for Ba8Au4.5Ge40.3□1.1 did not show any off-centering in this position.
References
G. Cordier and P. Woll, Neue ternäre intermetallische Verbindungen mit Clathratstruktur: Ba8(T,Si)6Si40 und Ba8(T,Ge)6Ge40 mit T = Ni, Pd, Pt, Cu, Ag, Au, J. Less-Common. Met., 1991, 169, p 291-302, in German
S. Johnsen, B. Thomsen, M. Christensen, G.K.H. Madsen, M. Nygren, and B. Iversen, An Exploration of Noble Metal Substitution in Germanium Based Clathrates, ICT, 2007, p 219
H. Anno, M. Hokazone, H. Takakura, and K. Matsubara, Thermoelectric Properties of Ba8Au x Ge46−x Clathrate Compounds, ICT, 2005, p 102
R.F.W. Herrmann, K. Tanigaki, T. Kawaguchi, S. Kuroshima, and O. Zhou, Electronic Structure of Si and Ge Gold-Doped clathrates, Phys. Rev. B, 1999 60(19), p 13245-13248
W. Carrillo-Cabrera, S. Budnyk, Y. Prots, and Y. Grin, Ba8Ge43 revisited: a 2a′ × 2a′ × 2a′ Superstructure of the Clathrate-I, Type with Full Vacancy Ordering, Z. Anorg. Allg. Chem., 2004, 630, p 7226
W. Carrillo-Cabrera, J. Curda, K. Peters, S. Paschen, M. Baenitz, Yu. Grin, and H.G. von Schnering, Crystal Structure of the Defect Clathrate-I, Ba8Ge43, Z. Kristallogr. NCS, 2000, 215, p 321
N.L. Okamoto, M.W. Oh, T. Nishii, K. Tanaka, and H. Inui, Crystal Structure and Thermoelectric Properties of the Type-I, Clathrate Compound Ba8Ge43 with an Ordered Arrangement of Ge Vacancies, J. Appl. Phys., 2006, 99, p 033513
N. May and H. Schäfer, Neue Verbindungen im ThCr2Si2-Typ, Z. Naturforsch., 1972, 27B, p 864, in German
N. Melnychenko-Koblyuk, A. Grytsiv, L. Fornasari, H. Kaldarar, H. Michor, F. Röhrbacher, M. Koza, E. Royanian, E. Bauer, P. Rogl, M. Rotter, H. Schmid, F. Marabelli, A. Devishvili, M. Doerr, and G. Giester, Ternary Clathrates Ba-Zn-Ge: Phase Equilibria, Crystal Chemistry and Physical Properties, J. Phys.: Condens. Matter, 2007, 19, p 216223
N. Melnychenko-Koblyuk, A. Grytsiv, G. Giester, E. Bauer, P. Rogl, M. Rotter, L. Lackner, L. Fornasari, and F. Marabelli, Structure and Physical Properties of Type-I, Clathrate Solid-Solution Ba8Pt x Ge46−x−y □ y (□ = vacancy), Phys. Rev. B, 2007, 76, p 195124
N. Melnychenko-Koblyuk, A. Grytsiv, G. Giester, St. Berger, H. Kaldarar, H. Michor, F. Röhrbacher, E. Royanian, E. Bauer, P. Rogl, M. Rotter, and H. Schmid, Ternary Clathrates Ba Cd Ge: Phase Equilibria, Crystal Chemistry and Physical Properties, J. Phys.: Condens. Matter, 2007, 19, p 046203
I. Zeiringer, M.X. Chen, I. Bednar, E. Royanian, E. Bauer, R. Podloucky, A. Grytsiv, P. Rogl, and H. Effenberger, Phase Equilibria, Crystal Chemistry, Electronic Structure and Physical Properties of Ag-Ba-Ge Clathrates, Acta. Mater., 2011, 59, p 2368-2384
N. Melnychenko-Koblyuk, A. Grytsiv, P. Rogl, and H. Schmid, The Clathrate Ba8Cu x Ge46−x−y □ y : Phase Equilibria and Crystal Structure, J. Solid State Chem., 2009, 182, p 1754
G. Bruzzone and G.B. Bonino, Alcuni composti intermetrallici M X2 formati dal Ca, Sr e Ba, Atti della Accademia Nazionale dei Lincei, Classe di Scienze Fisiche, Matematiche e Naturali, Rendiconti, 1970, 48, p 235-241, in Italian
I. Zeiringer, E. Bauer, A. Grytsiv, P. Rogl, and H. Effenberger, The Ternary System Au-Ba-Si: Clathrate Compounds and Physical Properties, Phase Equilibria and Crystal Structures; to be published
W. Bazela, The Influence of the Crystal Structure on the Magnetic Ordering in RT2X2 and RTX3 Compounds, J. Alloys Compd., 2007, 442, p 132-135
V.K. Pecharskii, O.I. Bodak, and Yu.V. Pankevich, The Crystal Structure of New Antimonides CeNi2Sb2 and CeNi2Sb2 p-Phase, Seriya B: Geologichni, Khimichni ta Biologichni Nauki, 1982, p 46-49
J. Callaway, Low-Temperature Lattice Thermal Conductivity, Phys. Rev., 1961, 122, p 787
D. Cahill and R. Pohl, Heat Flow and Lattice Vibrations in Glasses, Solid State Commun., 1989, 70, p 927
F.J. Blatt, Physics of Electronic Conduction in Solids, McGraw-Hill, New York, 1968, p 210
J.C.H. Chiu, Deviations from Linear Temperature Dependence of the Electrical Resistivity of V-Cr and Ta-W Alloys, Phys. Rev. B, 1976, 13, p 1507
S. Berger, “Novel Thermoelectric Materials,” Ph.D. thesis, TU Vienna, Austria, 2003
H. Zhang, H. Borrmann, N. Oeschler, Ch. Candolfi, W. Schnelle, M. Schmidt, U. Burkhardt, M. Baitinger, J.-T. Zhao, and Y. Grin, Atomic Interactions in the p-Type Clathrate I Ba8Au5.3Ge40.7, Inorg. Chem., 2011, 50, p 1250-1257
Acknowledgment
Work partially supported by the Austrian FFG Project “THECLA”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeiringer, I., Melnychenko-Koblyuk, N., Grytsiv, A. et al. Phase Equilibria, Crystal Chemistry and Physical Properties of Au-Ba-Ge Clathrates. J. Phase Equilib. Diffus. 32, 115–127 (2011). https://doi.org/10.1007/s11669-011-9852-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-011-9852-7