Abstract
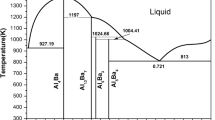
First-principles (FP) energetics of both the constituent elements and the compounds in the Al-Ca binary system are used in the CALPHAD (CALculation of PHase Diagrams) approach of thermodynamic modeling. First-principles calculations are performed using both an all-electron full-potential linearized augmented plane-wave method, as well as an ultrasoft pseudopotential/plane wave method. We perform calculations of T=0 ground state total energies of the pure Al and Ca in fcc, bcc, and hcp structures, and the binary compounds in their observed crystal structures. Al4Ca, Al14Ca13, and Al3Ca8 are modeled in CALPHAD as simple stoichiometric compounds; however, the Laves C15 compound, Al2Ca, is modeled using two sublattices (Al,Ca)2(Al,Ca)1, necessitating first-principles energies of both the stable Al2Ca compound as well as the three nonstable Al2Al, AlCa2, and Ca2Ca compounds. From these total energies, we obtain the formation enthalpies of all the binary compounds that are then used to assist in evaluating the Gibbs energy functions for the individual phases. The entropy contribution in the Gibbs energy function for each individual compound is obtained via the observed equilibria with the liquid phase. We provide a complete thermodynamic description of the Al-Ca binary system, evaluated by this combined CALPHAD-FP approach.
Similar content being viewed by others
References
K. Ozturk, L.Q. Chen, and Z.K. Liu: J. Alloys Compounds, 2002, vol. 340 pp. 199–206.
B.Q. Huang and J.D. Corbett: Inorg. Chem., 1998, vol. 37, pp. 5827–33.
D. Kevorkov and R. Schmid-Fetzer: Z. Metallkd., 2001, vol. 92, pp. 946–52.
M. Hansen and K. Anderko: Constitution of Binary Alloys, 2nd ed., McGraw-Hill, New York, NY, 1958.
V.P. Itkin, C.B. Alcock, P.J. van Ekeren, and H.A.J. Oonk: Bull. Alloy Phase Diagrams, 1988, vol. 9, pp. 652–57.
H. Nowotny and A. Mohrnheim: Z. Krist., 1939, vol. A100, pp. 540–42.
H. Nowotny, E. Wormnes, and A. Mohrnheim: Z. Metallkd., 1940, vol. 32, pp. 39–42.
D. Kevorkov, R. Schmid-Fetzer, A. Pisch, F. Hodaj, and C. Colinet: Z. Metallkd., 2001, vol. 92, pp. 953–58.
C. Wolverton, X.Y. Yan, R. Vijayaraghavan, and V. Ozolins: Acta Mater., 2002, vol. 50, pp. 2187–97.
B. Jansson: Trita-Mac-0234, Royal Institute of Technology, Stockholm, 1984.
G.K.H. Madsen, P. Blaha, K. Schwarz, E. Sjostedt, and L. Nordstrom: Phys. Rev. B, 2001, vol. 64, p. 195134.
E. Sjostedt, L. Nordstrom, and D.J. Singh: Solid State Commun., 2000, vol. 114, pp. 15–20.
P. Blaha, K. Schwarz, G.K.H. Madsen, D. Kvasnicka, and j. Luitz: WIEN2k: An Augmented Plane Wave + Local Orbitals Program for Calculating Crystal Properties, revised ed., Wien, Austria, 2001.
G. Kresse and J. Hafner: Phys. Rev. B, 1993, vol. 47, pp. 558–61.
G. Kresse: Thesis, Technische Universitat, Wien, 1993.
G. Kresse and J. Furthmuller: Phys. Rev. B, 1996, vol. 54, pp. 11169–11186.
G. Kresse and J. Furthmuller: Comput. Mater. Sci., 1996, vol. 6, pp. 15–50.
P. Hohenberg and W. Kohn: Phys. Rev., 1964, vol. 136B, pp. 864–71.
W. Kohn and L.J. Sham: Phys. Rev., 1965, vol. 140, pp. A1133-A1138.
J.P. Perdew and Y. Wang: Phys. Rev. B, 1992, vol. 45, pp. 13244–13249.
D.M. Ceperley and B.J. Alder: Phys. Rev. Lett., 1980, vol. 45, pp. 566–69.
J.P. Perdew, K. Burke, and M. Ernzerhof: Phys. Rev. Lett., 1996, vol. 77, pp. 3865–68.
J.P. Perdew and A. Zunger: Phys. Rev. B, 1981, vol. 23, pp. 5048–79.
J.P. Perdew, J.A. Chevary, S.H. Vosko, K.A. Jackson, M.R. Pederson, D.J. Singh, and C. Fiolhais: Phys. Rev. B, 1992, vol. 46, pp. 6671–87.
J.P. Perdew, J.A. Chevary, S.H. Vosko, K.A. Jackson, M.R. Pederson, D.J. Singh, and C. Fiolhais: Phys. Rev. B, 1993, vol. 48, p. 4978.
D. Vanderbilt: Phys. Rev. B, 1990, vol. 41, pp. 7892–95.
G. Kresse and J. Hafner: J. Phys.-Condes. Matter, 1994, vol. 6, pp. 8245–57.
H.J. Monkhorst and J.D. Pack: Phys. Rev. B, 1976, vol. 13, pp. 5188–92.
B. Sundman, B. Jansson, and J.O. Andersson: CALPHAD, 1985, vol. 9, pp. 153–90.
A.T. Dinsdale: CALPHAD, 1991, vol. 15, pp. 317–425.
A.T. Kister and O. Redlich: Ind. Eng. Chem., 1948, vol. 40, pp. 345–48.
I. Ansara, T.G. Chart, A.F. Guillermet, F.H. Hayes, U.R. Kattner, D.G. Pettifor, N. Saunders, and K. Zeng: CALPHAD, 1997, vol. 21, pp. 171–218.
Z.-K. Liu and Y.A. Chang: CALPHAD, 1999, vol. 23, pp. 339–56.
J.G.C. Neto, S.G. Fries, H.L. Lukas, S. Gama, and G. Effenberg: CALPHAD, 1993, vol. 17, pp. 219–28.
K.J. Zeng, M. Hamalainen, and R. Luoma: Z. Metallkd., 1993, vol. 84, pp. 23–28.
J.C. Boettger and S.B. Trickey: Phys. Rev. B, 1996, vol. 53, pp. 3007–12.
W. Witt: Z. Naturforsch. A, 1967, vol. 22, pp. 92–95.
B.T. Bernstein and J.F. Smith: Acta Crystallogr., 1959, vol. 12, pp. 419–20.
M. Notin, J. Mejbar, A. Bouhajib, J. Charles, and J. Hertz: J. Alloys Compounds, 1995, vol. 220, pp. 62–75.
E. Veleckis: J. Less-Common Met., 1981, vol. 80, pp. 241–55.
M. Notin and J. Hertz: CALPHAD, 1982, vol. 6, pp. 49–56.
Y. Zhong, C. Wolverton, Y.A. Chang, and Z.-K. Liu: Acta Mater., 2004, vol. 52, pp. 2739–54.
K. Matsuyama: Sci. Rep. Tohoku Univ., 1928, vol. 17, pp. 783–89.
L. Donski: Z. Anorg. Chem., 1908, vol. 57, pp. 201–05.
G. Bozza and C. Sonnino: Giorn. Chim. Ind. Appl., 1928, vol. 10, pp. 443–49.
M. Notin, J.C. Gachon, and J. Hertz: J. Less-Common Met., 1982, vol. 85, pp. 205–12.
M. Notin, J.C. Gachon, and J. Hertz: J. Chem. Thermodyn., 1982, vol. 14, pp. 425–34.
F. Sommer, J.J. Lee, and B. Predel: Z. Metallkd., 1983, vol. 74, pp. 100–04.
K.T. Jacob, S. Srikanth, and Y. Waseda: Trans. Jpn. Inst. Met., 1988, vol. 29, pp. 50–59.
E. Schurmann, C.P. Funders, and H. Litterscheidt: Arch. Eisenhuttenwes., 1975, vol. 46, pp. 473–76.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ozturk, K., Zhong, Y., Chen, LQ. et al. Linking first-principles energetics to CALPHAD: An application to thermodynamic modeling of the Al-Ca binary system. Metall Mater Trans A 36, 5–13 (2005). https://doi.org/10.1007/s11661-005-0133-0
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11661-005-0133-0