Summary
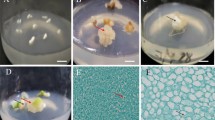
Mature embryo axes of the Ohio buckeye were germinated on a medium containing 1 mg gibberellic acid (GA) per 1. Three wk following germination, stem, petiole, and leaf blade tissues were excised and placed on media containing either 1 mg (4.5 µM) 2,4-dichlorophenoxy acetic acid (2,4-D) per 1, 1 mg (4.7 µM) kinetin per 1, 1 mg of both 2,4-D (4.5 µM) and kinetin (4.7 µM per 1, or 2 mg of both 2,4-D (9.1 µM) and kinetin (9.3 µM) per 1. Embryogenic tissue was formed only from stem segments after 2–3 mo. of culture on media containing both 2,4-D and kinetin. Embryogenic tissue could be either maintained on solid medium for proliferation of embryogenic callus or placed in liquid medium for proliferation of embryogenic suspension cultures. For transformation of suspension cultures, tissues were inoculated with Agrobacterium EHA105 containing the binary plasmid Vec035, briefly sonicated, and cultured in the presence of 100 µM acetosyringone for 2 d. To eliminate Agrobacterium, tissues were washed and placed in liquid proliferation medium containing either 500 mg Cefotaxime per 1 or 400 mg TimentinŖ per 1. Selection on 20 mg hygromycin per 1 was initiated 2 wk after inoculation, and after an additional 10 wk, hygromycin-resistant tissue was isolated and separately cultured. Although some hygromycinresistant clones were recovered with no sonication treatment, four to five times more clones were obtained following sonication. Putative transformed clones were confirmed to be transgenic via both histochemical β-glucuronidase (GUS) assay and southern hybridization analyses. Development of transgenic embryos occurred on a growth regulator-free medium containing 3% sucrose. After 2 mo. of embryo development, the embryos were transferred to fresh medium for germination.
Similar content being viewed by others
References
Bergmann, B. A.; Hackett, W. P.; Pellett, H. Somatic embryogenesis in Aesculus. In Vitro Cell. Dev. Biol. Plant 32:161–164; 1996.
Bommineni, V. R.; Chibbar, R. N.; Datla, R. S. S.; Tsang, E. W. T. Transformation of white spruce (Picea glauca) somatic embryos by microprojectile bombardment. Plant Cell Rep. 13:17–23; 1993.
Cabrera-Ponce, J. L.; Vegas-Garcia, A.; Herrera-Estrella, L. Regeneration of transgenic papaya plants via somatic embryogenesis induced by Agrobacterium rhizogenes. In Vitro Cell. Dev. Biol. Plant 32:86–90; 1996.
Chee, P. P. Plant regeneration from somatic embryos of Taxus brevifolia. Plant Cell Rep. 16:184–187; 1996.
Cheng, Y. H.; Yang, J. S.; Yeh, S. D. Efficient transformation of papaya by coat protein gene of papaya ringspot virus mediated by Agrobacterium following liquid-phase wounding by embryogenic tissues with carborundum. Plant Cell Rep. 16:127–132; 1996.
Dameri, R. M.; Caffaro, L.; Gastaldo, P.; Profumo, P. Callus formation and embryogenesis with leaf explants of Aesculus hippocastanum L. J. Plant Physiol. 126:93–96; 1986.
Denchev, P. D.; Atanassov, A. I. Micropropagation through somatic embryos. In: Biotechnology in agriculture and forestry: somatic embryogenesis and synthetic seed. Berlin, New York: Springer-Verlag; 1995:193–206.
Finer, J. J. Direct somatic embryogenesis. In: Gamborg, O. L.; Phillips, G. C., ed. Plant cell, tissue and organ culture—fundamental methods. Berlin: Springer-Verlag; 1995:91–102.
Finer, J. J.; McMullen, M. D. Transformation of cotton (Gossypium hirsutum L.) via particle bombardment. Plant Cell Rep. 8:586–590; 1990.
Finer, J. J.; McMullen, M. D. Transformation of soybean via particle bombardment of embryogenic suspension culture tissue. In Vitro Cell. Dev. Biol. Plant 27:175–182; 1991.
Finer, J. J.; Nagasawa, A. Development of an embryogenic suspension culture of soybean [Glycine max (L.) Merrill]. Plant Cell Tissue Organ Cult. 15:125–136; 1988.
Gamborg, O. L.; Miller, R. A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 50:150–158; 1968.
Gastaldo, P.; Carli, S.; Profumo, P. Somatic embryogenesis from stem explants of Aesculus hippocastanum. Plant Cell Tissue Organ Cult. 39:97–99; 1994.
Hansen, G.; Das, A.; Chilton, M. Constitutive expression of the virulence genes improves the efficiency of plant transformation by Agrobacterium. Proc. Natl. Acad. Sci. USA 91:7603–7607; 1994.
Hiei, Y.; Ohta, S.; Komari, T.; Kumatshiro, T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 6:271–282; 1994.
Hood, E. E.; Gelvin, S. B.; Melchers, L. S.; Hoekema, A. New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res. 2:208–218; 1993.
Ishida, Y.; Saito, H.; Ohta, S.; Hiei, Y.; Komari, T.; Kumashiro, T. High efficiency transformation of maize (Zea mays L.) mediated by Agrobacterium tumefaciens. Nat. Biotechnol. 14:745–750; 1996.
Jefferson, R. A. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol. Biol. Rep. 5:387–405; 1987.
Komari, T. Transformation of cultured cells of Chenopodium quinoa by binary vectors that carry a fragment of DNA from the virulence region of pTiBo542. Plant Cell Rep. 9:303–306; 1990.
McGranahan, G.; Leslie, C.; Uratsu, S.; Martin, L.; Dandekar, A. Agrobacterium-mediated transformation of walnut somatic embryos and regeneration of transgenic plants. Bio/Technology 6:800–804; 1988.
Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473–497; 1962.
Radojevic, L. Plant regeneration of Aesculus hippocastanum L. (Horse Chestnut) through somatic embryogenesis. J. Plant Physiol. 132:322–326; 1988.
Saghei-Maroof, M. A.; Saliman, K. M.; Jorgensen, R. A.; Wallard, R. Ribosomal DNA spacer length polymorphism in barley: Mendelian inheritance, chromosomal location and population dynamics. Proc. Natl. Acad. Sci. USA 81:8014–8018; 1984.
Stachel, S.; Messens, E.; Van Montagu, M.; Zambryski, P. Identification of the signal molecules produced by wounded plant cells which activate the T-DNA transfer process in Agrobacterium tumefaciens. Nature (Lond.) 318:624–629; 1985.
Sullivan, J.; Lagrimini, L. M. Transformation of Liquidambar styraciflua using Agrobacterium tumefaciens. Plant Cell Rep. 12:303–306; 1993.
Tinland, B.; Hohn, B. Recombination between prokaryotic and eukaryotic DNA: integration of Agrobacterium tumefaciens T-DNA into the plant genome. In: Setlow, J. K., ed. Genetic engineering. Vol. 17. New York: Plenum Press; 1995:209–229.
Torisky, R. S.; Kovacs, L.; Avdiushko, S.; Newman, J. D.; Hunt, A. G.; Collins, G. B. Development of a binary vector system for plant transformation based on the supervirulent Agrobacterium tumefaciens strain Chry5. Plant Cell Rep. 17:102–108; 1997.
Trick, H. N.; Finer, J. J. SAAT: sonication assisted Agrobacterium-mediated transformation. Transgenic Res. 6:329–334; 1997.
Trick, H. N.; Finer, J. J. Sonication-assisted Agrobacterium-mediated transformation of soybean (Glycine max [L.] Merr.) embryogenic suspension tissue cultures. Plant Cell Rep. 17:482–488; 1998.
Wilde, H. D.; Merkle, S. A. Genetic transformation in Liriodendron tulipifera L. (yellow poplar). In: Bajaj, Y. P. S., ed. Plant protoplasts and genetic engineering. Biotechnol. Agric. For. Berlin: Springer-Verlag 29:337–348; 1994.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Trick, H.N., Finer, J.J. Induction of somatic embryogenesis and genetic transformation of ohio buckeye (Aesculus glabra willd.). In Vitro Cell.Dev.Biol.-Plant 35, 57–60 (1999). https://doi.org/10.1007/s11627-999-0010-4
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11627-999-0010-4