Abstract
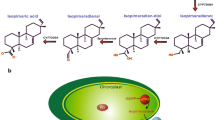
Benzylisoquinoline alkaloids are a large and structurally diverse group of natural plant products that includes many compounds with potent biological activities, including the antimicrobial agent sanguinarine. The putative subcellular localization of the sanguinarine pathway was determined using in-frame N-terminal fusions between cDNAs encoding nine consecutive biosynthetic enzymes and the gene encoding the green fluorescent protein (GFP). Expression constructs were introduced into cultured opium poppy cells by particle bombardment, and the localization of fusion proteins was visualized using epifluorescence microscopy. GFP fusions with two O-methyltransferases and two N-methyltransferases in the sanguinarine pathway all produced non-targeted fluorescence in the cytosol and nucleus. Interspersed between these soluble proteins are five membrane-bound cytochromes P450. Corresponding cDNAs are available for three P450s, all of which produced fluorescence when fused to GFP in association with the endoplasmic reticulum (ER). Two enzymes with suggested or known N-terminal signal peptides were initially associated with the ER, but were subsequently transported to the central vacuole suggesting their occurrence in the ER lumen. The alternating localization of these biosynthetic enzymes to three subcellular compartments indicates extensive trafficking of pathway intermediates across the endomembranes and suggests a key role for compartmentalization in the regulation of sanguinarine metabolism.





Similar content being viewed by others
References
Alcantara J, Bird DA, Franceschi VR, Facchini PJ (2005) Sanguinarine biosynthesis is associated with the endoplasmic reticulum in cultured opium poppy cells after elicitor treatment. Plant Physiol 138:173–183
Allen RS, Miller JAC, Chitty JA, Fist AJ, Gerlach WL, Larkin PJ (2008) Metabolic engineering of morphinan alkaloids by over-expression and RNAi suppression of salutaridinol 7-O-acetyltransferase in opium poppy. Plant Biotech J 6:22–30
Amann M, Wanner G, Zenk MH (1986) Intracellular compartmentation of two enzymes of berberine biosynthesis in plant cell cultures. Planta 167:310–320
Angelova S, Buchheim M, Frowitter D, Schierhorn A, Roos W (2010) Overproduction of alkaloid phytoalexins in California poppy cells is associated with the co-expression of biosynthetic and stress-protective enzymes. Mol Plant 3:927–939
Arakawa H, Clark WG, Psenak M, Coscia CJ (1992) Purification and characterization of dihydrobenzophenanthridine oxidase from elicited Sanguinaria canadensis cell culture. Arch Biochem Biophys 299:1–7
Bauer W, Zenk MH (1991) Two methylenedioxy bridge forming cytochrome P-450 dependent enzymes are involved in (S)-stylopine biosynthesis. Phytochemistry 30:2953–2962
Bird DA, Facchini PJ (2001) Berberine bridge enzyme, a key branch-point enzyme in benzylisoquinoline alkaloid biosynthesis, contains a vacuolar-sorting determinant. Planta 213:888–897
Bock A, Wanner G, Zenk MH (2002) Immunocytological localization of two enzymes involved in berberine biosynthesis. Planta 216:57–63
Deus-Neumann B, Zenk MH (1984) A highly selective alkaloid uptake system in vacuoles of higher plants. Planta 162:250–260
Deus-Neumann B, Zenk MH (1986) Accumulation of alkaloids in plant vacuoles does not involve an ion-trap mechanism. Planta 167:44–53
Facchini PJ, Johnson AG, Poupart J, De Luca V (1996a) Uncoupled defense gene expression and antimicrobial alkaloid accumulation in elicited opium poppy cell cultures. Plant Physiol 111:687–697
Facchini PJ, Penzes C, Johnson AG, Bull D (1996b) Molecular characterization of berberine bridge enzyme genes from opium poppy. Plant Physiol 112:1669–1677
Gamborg O, Miller R, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Ikezawa N, Iwasa K, Sato F (2007) Molecular cloning and characterization of methylenedioxy bridge-forming enzymes involved in stylopine biosynthesis in Eschscholzia californica. FEBS J 274:1019–1035
Ikezawa N, Iwasa K, Sato F (2009) CYP719A subfamily of cytochrome P450 oxygenases and isoquinoline alkaloid biosynthesis in Eschscholzia californica. Plant Cell Rep 28:123–133
Kakiuchi N, Hattori M, Ishii H, Namba T (1987) Effect of benzo[c]phenanthridine alkaloids on reverse transcriptase and their binding property to nucleic acids. Planta Med 53:22–27
Kempe K, Higashi Y, Frick S, Sabarna K, Kutchan TM (2009) RNAi suppression of the morphine biosynthetic gene salAT and evidence of association of pathway enzymes. Phytochemistry 70:579–589
Lee E-J, Facchini PJ (2010) Norcoclaurine synthase is a member of the pathogenesis-related 10/Bet v1 protein family. Plant Cell 22:3489–3503
Leonard E, Koffas MAG (2007) Engineering of artificial plant cytochrome P450 enzymes for synthesis of isoflavones by Escherichia coli. Appl Environ Microbiol 73:7246–7251
Liscombe DK, Facchini PJ (2007) Molecular cloning and characterization of tetrahydroprotoberberine cis-N-methyltransferase, an enzyme involved in alkaloid biosynthesis in opium poppy. J Biol Chem 282:14741–14751
Liscombe DK, MacLeod BP, Loukanina N, Nandi OI, Facchini PJ (2005) Evidence for the monophyletic evolution of benzylisoquinoline alkaloid biosynthesis in angiosperms. Phytochemistry 66:2500–2520
Otani M, Shitan N, Sakai K, Martinoia E, Sato F, Yazaki K (2005) Characterization of vacuolar transport of the endogenous alkaloid berberine in Coptis japonica. Plant Physiol 138:1939–1946
Rueffer M, Zenk MH (1987) Enzymatic formation of protopines by a microsomal cytochrome P450 system of Corydalis vaginans. Tetrahedron Lett 28:5307–5310
Samanani N, Liscombe DK, Facchini PJ (2004) Molecular cloning and characterization of norcoclaurine synthase, an enzyme catalyzing the first committed step in benzylisoquinoline alkaloid biosynthesis. Plant J 40:302–313
Schmeller T, Latz-Brüning B, Wink M (1997) Biochemical activities of berberine, palmitine and sanguinarine mediating chemical defense against microorganisms and herbivores. Phytochemistry 44:257–266
Schumacher H-M, Zenk MH (1988) Partial purification and characterization of dihydrobenzophenanthridine oxidase from Eschscholzia californica cell suspension cultures. Plant Cell Rep 7:43–46
Seibel NM, Eljouni J, Nalaskowski MM, Hampe W (2007) Nuclear localization of enhanced green fluorescent protein homomultimers. Anal Biochem 368:95–99
Shitan N, Bazin I, Dan K, Obata K, Kigawa K, Ueda K, Sato F, Forestier C, Yazaki K (2003) Involvement of CjMDR1, a plant multidrug-resistance-type ATP-binding cassette protein, in alkaloid transport in Coptis japonica. Proc Natl Acad Sci USA 100:751–756
Shockey JM, Gidda SK, Chapital DC, Kuan J-C, Dhanoa PK, Bland JM, Rothstein SJ, Mullen RT, Dyer JM (2006) Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell 18:2294–2323
Tanahashi T, Zenk MH (1990) Elicitor induction and characterization of microsomal protopine-6-hydroxylase, the central enzyme in benzophenanthridine alkaloid biosynthesis. Phytochemistry 29:1113–1122
Vavreckova C, Gawlik I, Muller K (1996) Benzophenanthridine alkaloids of Chelidonium majus. 1. Inhibition of 5- and 12-lipoxygenase by a non-redox mechanism. Planta Med 62:397–401
Vogel M, Lawson M, Sippl W, Conrad U, Roos W (2010) Structure and mechanism of sanguinarine reductase, an enzyme of alkaloid detoxification. J Biol Chem 285:18397–18406
Weiss D, Baumert A, Vogel M, Roos W (2006) Sanguinarine reductase, a key enzyme of benzophenanthridine detoxification. Plant Cell Environ 29:291–302
Wolff J, Knipling L (1993) Antimicrotubule properties of benzophenanthridine alkaloids. Biochemistry 32:13334–13339
Ziegler J, Facchini PJ (2008) Alkaloid biosynthesis: metabolism and trafficking. Ann Rev Plant Biol 59:735–769
Zulak KG, Cornish A, Daskalchuk TE, Deyholos MK, Goodenowe DB, Gordon PMK, Klassen D, Pelcher LE, Sensen CW, Facchini PJ (2007) Gene transcript and metabolite profiling of elicitor-induced opium poppy cell cultures reveals the coordinate regulation of primary and secondary metabolism. Planta 225:1085–1106
Acknowledgments
We thank Dr. Richard Bourgault for assistance with the construction of plasmid vectors, Dr. Nailish Samanani for assistance with the microprojectile bombardment, and Dr. Robert Mullen (University of Guelph) for providing the pRFP-HDEL construct. P.J.F. holds the Canada Research Chair in Plant Metabolic Processes Biotechnology. This work was funded by the Natural Sciences and Engineering Research Council of Canada Discovery Grant to P.J.F..
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Forster
Rights and permissions
About this article
Cite this article
Hagel, J.M., Facchini, P.J. Subcellular localization of sanguinarine biosynthetic enzymes in cultured opium poppy cells. In Vitro Cell.Dev.Biol.-Plant 48, 233–240 (2012). https://doi.org/10.1007/s11627-012-9426-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-012-9426-3