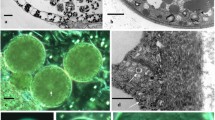
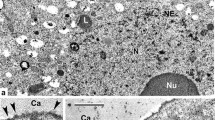
Abstract
Pachyphlodes (Pezizaceae) is a genus of truffle-like fungi that is distributed across the Northern Hemisphere. These fungi form ectomycorrhizae primarily with trees in the Fagaceae family, and occasionally with other host plants. The genus Plicariella (= Scabropezia) is phylogenetically inferred as an ally of, or within, the Pachyphlodes lineage. Despite molecular phylogenetic analyses that show the close relationships of species in these two genera, morphological differences in ascomata shape and color, spore ornamentation, and ascus shape are profound. Here, we studied spore wall development to better understand affinities within the Pachyphlodes–Plicariella lineages. Electron microscopy studies indicate that the initial spore wall development is similar across six Pachyphlodes species and a Plicariella species, despite striking differences in mature spore ornamentation among species. Ultrastructural analyses reveal that differences in spore ornamentation among Pachyphlodes species are due to unique developmental events at the final stages of spore wall deposition. Septal pore ultrastructure in Pachyphlodes species is similar to other Pezizaceae that have been studied. Molecular analyses of the five species studied indicate that four of them have not been previously described. The new species Pachyphlodes annagardnerae is here described, and the ultrastructural features of species of Pachyphlodes, Plicariella, and other Pezizales are compared and discussed.







Similar content being viewed by others
References
Araujo JC, Téran FC, Oliveira RA, Nour EAA, Montenegro MAP, Campos JR, Vazoller RF (2003) Comparison of hexamethyldisilazane and critical point drying treatments for SEM analysis of anaerobic biofilms and granular sludge. J Electron Microsc 52:429–433
Arora D (1986) Mushrooms demystified. Ten Speed Press, Berkeley
Bellemère A (1994) Asci and ascospores in Ascomycete systematics. In: Hawksworth DL (ed) Ascomycete systematics: problems and perspectives in the nineties. Plenum Press, New York, pp 111–126
Berkeley MJ, Broome CE (1846) Notices of British hypogeous fungi. Ann Mag Nat Hist 18:73–82
Bóka K, Király I, Bratek Z (1992) Ultrastructural observations on ascosporogenesis of Pachyphloeus melanoxanthus. Micologia Vegetazione Mediterranea 7:57–64
Bonito G, Brenneman T, Vilgalys R (2011) Ectomycorrhizal fungal diversity in orchards of cultivated pecan (Carya illinoinensis; Juglandaceae). Mycorrhiza 21:601–612
Corda ACJ (1854) Icones fungorum hucusque cognitorum 6:55
Curry KJ, Kimbrough JW (1983) Septal structures in apothecial tissues of the Pezizaceae (Pezizales, Ascomycetes). Mycologia 75:781–794
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772
Dissing H, Pfister DH (1981) Scabropezia, a new genus of Pezizaceae (Pezizales). Nord J Bot 1:102–108
Doweld AB (2013a) Nomenclatural novelties. Index Fungorum 31:1
Doweld AB (2013b) Nomenclatural novelties. Index Fungorum 32:1
Dyby SD, Kimbrough JW (1987) A comparative ultrastructural study of ascospore ontogeny in selected species of Peziza (Pezizales; Ascomycetes). Bot Gaz 148:283–296
Exbrayat J-M (2010) Cytochemical techniques. In: Exbrayat J-M (ed) Histochemical and cytochemical methods of visualization. CRC Press, Boca Raton
Frank JL, Barry S, Southworth D (2006) Mammal mycophagy and dispersal of mycorrhizal inoculum in Oregon white oak woodlands. Northwest Sci 80:264–273
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Gilkey HM (1916) A revision of the Tuberales of California. Univ Cal Publ Bot 6:275–356, pls. 26–30
Gilkey HM (1939) Tuberales of North America. Stud Bot 1:1–63
Glenn TC, Schable NA (2005) Isolating microsatellite DNA loci. Methods Enzymol 395:202–222
Göppert HR (1836) Die fossilen Farrenkräuter (Systema filicum fossilum). Nova Acta Leopoldina 17:1–486, pls. 1–44
Guindon S, Gascuel O (2003) A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Syst Biol 52:696–704
Handley PS, Hargreaves J, Harty DWS (1988) Ruthenium red staining reveals surface fibrils and a layer external to the cell wall in Streptococcus salivarius HB and adhesion deficient mutants. J Gen Microbiol 134:3165–3172
Hansen K, LoBuglio KF, Pfister DH (2005) Evolutionary relationships of the cup-fungus genus Peziza and Pezizaceae inferred from multiple nuclear genes: RPB2, β-tubulin, and LSU rDNA. Mol Phylogenet Evol 36:1–23
Harkness HW (1899) Californian hypogaeous fungi. Proc Cal Acad Sci Ser 3 1(8):241–293
Hayat MA (1981) Fixation for electron microscopy. Academic Press, London
Healy RA (2003) Mattirolomyces tiffanyae, a new truffle from Iowa, with ultrastructural evidence for its classification in the Pezizaceae. Mycologia 95:765–772
Healy RA (2013) Molecular systematics and morphological congruence in the Pezizales and Neolectales (Ascomycota): three case studies. Dissertation, University of Minnesota
Healy RA, Bonito G, Guevara G (2009) The truffle genus Pachyphloeus in the U.S. and Mexico: phylogenetic analysis and a new species. Mycotaxon 107:61–71
Healy RA, Smith ME, Bonito GM, Pfister DH, Ge ZW, Guevara GG, Williams G, Stafford K, Kumar L, Lee T, Hobart C, Trappe J, Vilgalys R, McLaughlin DJ (2013) High diversity and widespread occurrence of mitotic spore mats in ectomycorrhizal Pezizales. Mol Ecol 22:1717–1732
Healy RA, Hobart C, Tocci GE, Bóna L, Merényi Z, Paz Conde A, Smith ME (2015) Fun with the discomycetes: revisiting collections of Korf’s anamorphic Pezizales and Thaxter’s New England truffles leads to a connection between forms and the description of two new truffle species: Pachyphlodes pfisteri and P. nemoralis. Ascomycete.org 7:357–366
Hirsch G (1985) The genera Scabropezia and Plicaria in the German Democratic Republic. Agarica 6:241–258
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755
Janex-Favre MC, Parguey-Leduc A (1980) Formation et évolution des ascospores du Tuber mesentericum Vitt. Bulletin trimestriel de la Sociéte Mycologique de France 96:225–237
Janex-Favre MC, Parguey-Leduc A (1983) Étude ultrastructurale des asques et des ascospores de truffes du genre Tuber. II. Les ascospores. Cryptogam Mycol 4:353–373
Janex-Favre MC, Parguey-Leduc A (1985) Les asques et les ascospores du Terfezia claveryi Ch. (Tubérales). Cryptogam Mycol 6:87–99
Janex-Favre MC, Parguey-Leduc A, Riousset L (1988) L’ascocarpe hypoge d’une Terfez Francaise (Terfezia leptoderma Tul., Tuberales, Discomycètes). Bulletin trimestriel de la Sociéte Mycologique de France 104:145–178
Katoh K, Toh H (2010) Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics 26:1899–1900
Katoh K, Kuma K, Toh H, Miyata T (2005) MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res 33:511–518
Kelly KL, Judd DB (1955) The ISCC-NBS method of designating colors and a dictionary of color names. National Bureau of Standards circular 553, Washington, DC, 158 pp
Kimbrough JW (1994) Septal ultrastructure and ascomycete systematics. In: Hawksworth DL (ed) Ascomycete systematics: problems and perspectives in the nineties. Plenum Press, New York, pp 127–141
Kimbrough JW, Curry KJ (1985) Septal ultrastructure in the Ascobolaceae (Pezizales, Discomycetes). Mycologia 77:219–229
Kimbrough JW, Curry KJ (1986a) Septal structures in apothecial tissues of the tribe Aleurieae in the Pyronemataceae (Pezizales, Ascomycetes). Mycologia 78:407–417
Kimbrough JW, Curry KJ (1986b) Septal structures in apothecial tissues of taxa in the tribes Scutellinieae and Sowerbyelleae (Pyronemataceae, Pezizales, Ascomycetes). Mycologia 78:735–743
Kimbrough JW, Wu CG, Gibson JL (1991) Ultrastructural evidence for a phylogenetic linkage of the truffle genus Hydnobolites to the Pezizaceae (Pezizales, Ascomycetes). Bot Gaz 152:408–420
Kumar TKA, Healy RA, Spatafora JW, Blackwell M, McLaughlin DJ (2012) Orbilia ultrastructure, character evolution and phylogeny of Pezizomycotina. Mycologia 104:462–476
Læssøe T, Hansen K (2007) Truffle trouble: what happened to the Tuberales? Mycol Res 111:1075–1099
Lantieri A, Smith ME, Pfister DH (2012) A new species of Ruhlandiella (Pezizaceae) from Italy. Mycol Prog 11:509–513
Larget B, Simon DL (1999) Markov chain Monte Carlo algorithms for the Bayesian analysis of phylogenetic trees. Mol Biol Evol 16:750–759
Li LT (1997) Ultrastructural studies of Leucangium carthusianum (hypogeous Pezizales). Int J Plant Sci 158:189–197
Li LT, Kimbrough JW (1995) Spore wall ontogeny in Pseudoplectania nigrella and Plectania nannfeldtii (Ascomycotina, Pezizales). Can J Bot 73:1761–1767
Lindner DL, Banik MT (2009) Effects of cloning and root-tip size on observations of fungal ITS sequences from Picea glauca roots. Mycologia 101:157–165
Markham P (1994) Occlusions of septal pores in filamentous fungi. Mycol Res 98:1089–1106
McNeill J, Barrie FR, Buck WR, Demoulin V, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Marhold K, Prado J, Prud’Homme Van Reine WF, Smith GF, Wiersema JH, Turland NJ (2012) International code of nomenclature for algae, fungi, and plants (Melbourne code). Regnum Vegetabile 154. Koeltz Scientific Books
Merkus E (1975) Ultrastructure of the ascospore wall in Pezizales (Ascomycetes)—III. Otideaceae and Pezizaceae. Persoonia 8:227–247
Merkus E (1976) Ultrastructure of the ascospore wall in Pezizales (Ascomycetes)—IV. Morchellaceae, Helvellaceae, Rhizinaceae, Thelebolaceae, and Sarcoscyphaceae. General discussion. Persoonia 9:1–38
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the gateway computing environments workshop (GCE), New Orleans, LA, 14 November 2010, pp 1–8
Morris MH, Perez-Perez MA, Smith ME, Bledsoe CS (2009) Influence of host species on ectomycorrhizal communities associated with two co-occurring oaks (Quercus spp.) in a tropical cloud forest. FEMS Microbiol Ecol 69:274–287
Norman JE, Egger KN (1999) Molecular phylogenetic analysis of Peziza and related genera. Mycologia 91:820–829
Parguey-Leduc A, Janex-Favre MC, Montant C (1987) Formation et évolution des ascospores de Tuber melanosporum (truffe noire du Périgord, Discomycètes). Can J Bot 65:1491–1503
Pfister DH (1973a) The psilopezioid fungi. III. The genus Psilopezia (Pezizales). Am J Bot 60:355–365
Pfister DH (1973b) The psilopezioid fungi. IV. The genus Pachyella (Pezizales). Can J Bot 51:2009–2023
Rambaut A (2007) Se-Al: sequence alignment editor. Available online at: http://tree.bio.ed.ac.uk/software/seal
Rambaut A, Drummond AJ (2007) Tracer v1.4. Available online at: http://tree.bio.ed.ac.uk/software/tracer
Read ND, Beckett A (1996) Ascus and ascospore morphogenesis. Mycol Res 100:1281–1314
Ridgway R (1912) Color standards and color nomenclature. Published privately, Washington, DC, 43 pp, 53 pls
Smith ME, Douhan GW, Rizzo DM (2007) Ectomycorrhizal community structure in a xeric Quercus woodland based on rDNA sequence analysis of sporocarps and pooled roots. New Phytol 174:847–863
Spooner BM (2001) Plicaria (Pezizales) in Britain, and Plicariella reinstated. Czech Mycol 52:259–265
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690
Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for the RAxML web servers. Syst Biol 57:758–771
Stefani FOP, Moncalvo J-M, Séguin A, Bérubé JA, Hamelin RC (2009) Impact of an 8-year-old transgenic poplar plantation on the ectomycorrhizal fungal community. Appl Environ Microbiol 75:7527–7536
Tedersoo L, Suvi T, Jairus T, Ostonen I, Põlme S (2009) Revisiting ectomycorrhizal fungi of the genus Alnus: differential host specificity, diversity and determinants of the fungal community. New Phytol 182:727–735
Tedersoo L, May TW, Smith M (2010) Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 20:217–263
Tulasne LR, Tulasne C (1844) Fungi nonnulli hypogaei, novi v. minus cogniti auct. Giornale Botanico Italiano 2:55–63
Tulasne LR, Tulasne C (1851) Fungi Hypogaei: Histoire et Monographie des Champignons Hypogés. F. Klincksieck, Paris
Van Vooren N, Moyne G (2012) Plicariella flavovirens comb. nov. (Ascomycota, Pezizales), une pézize remarquable. Ascomycete.org 4:11–14
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172:4238–4246
Wells K (1972) Light and electron microscopic studies of Ascobolus stercorarius. II. Ascus and ascospore ontogeny. Univ Cal Publ Bot 62:1–93
White TJ, Bruns T, Lee SJ, Taylor JL (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Acknowledgements
We thank the following herbaria and their curators for specimen loans that were instrumental to this study: BG, CUP, FH, K, MA, NY, OSC, S, and SOC. The FLAS herbarium provided critical support for this work. Richard Kay generously translated the Latin description of P. ligericus. The following people contributed specimens and samples that were used in this study: Julio Cabero, Michael Castellano, Efren Cázares, Aurelia Paz Conde, Roy Halling, Carol Hobart, Debbie Klein, Alicia Knudson, Esther McLaughlin, Jean-Baptiste Perez, Branislav Peric, Lois H. Tiffany, and Nicolas Van Vooren. Lois Tiffany was a valued mentor for the ultrastructural portion of this project. Funding for RAH came from the Gilman Fund at Iowa State University, the Iowa Science Foundation, the Iowa Department of Natural Resources, the Minnesota Department of Natural Resources, The Society of Systematic Biologists, and The University of Minnesota Doctoral Dissertation Fellowship. Funding for MES was provided by NSF grant DEB-1354802, with additional support from the Institute for Food and Agricultural Sciences (IFAS) at the University of Florida.
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editors: Teresa Iturriaga and Marc Stadler
This article is part of the “Special Issue on ascomycete systematics in honour of Richard P. Korf who died in August 2016”.
Rights and permissions
About this article
Cite this article
Healy, R.A., Horner, H.T., Bonito, G. et al. An ultrastructural study of spore wall development and septal pores in species of the Pachyphlodes (Pezizaceae, Pezizales) lineage, with a description of the new species Pachyphlodes annagardnerae . Mycol Progress 17, 45–63 (2018). https://doi.org/10.1007/s11557-017-1348-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-017-1348-3