Abstract
Purpose
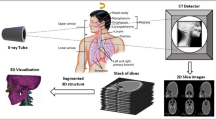
Minimally invasive alternatives are now available for many complex surgeries. These approaches are enabled by the increasing availability of intra-operative image guidance. Yet, fluoroscopic X-rays suffer from projective transformation and thus cannot provide direct views onto anatomy. Surgeons could highly benefit from additional information, such as the anatomical landmark locations in the projections, to support intra-operative decision making. However, detecting landmarks is challenging since the viewing direction changes substantially between views leading to varying appearance of the same landmark. Therefore, and to the best of our knowledge, view-independent anatomical landmark detection has not been investigated yet.
Methods
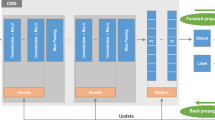
In this work, we propose a novel approach to detect multiple anatomical landmarks in X-ray images from arbitrary viewing directions. To this end, a sequential prediction framework based on convolutional neural networks is employed to simultaneously regress all landmark locations. For training, synthetic X-rays are generated with a physically accurate forward model that allows direct application of the trained model to real X-ray images of the pelvis. View invariance is achieved via data augmentation by sampling viewing angles on a spherical segment of \(120^\circ \times 90^\circ \).
Results
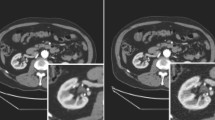
On synthetic data, a mean prediction error of 5.6 ± 4.5 mm is achieved. Further, we demonstrate that the trained model can be directly applied to real X-rays and show that these detections define correspondences to a respective CT volume, which allows for analytic estimation of the 11 degree of freedom projective mapping.
Conclusion
We present the first tool to detect anatomical landmarks in X-ray images independent of their viewing direction. Access to this information during surgery may benefit decision making and constitutes a first step toward global initialization of 2D/3D registration without the need of calibration. As such, the proposed concept has a strong prospect to facilitate and enhance applications and methods in the realm of image-guided surgery.









Similar content being viewed by others
References
Aichert A, Berger M, Wang J, Maass N, Doerfler A, Hornegger J, Maier AK (2015) Epipolar consistency in transmission imaging. IEEE Trans Med Image 34(11):2205–2219
Baumgartner R, Libuit K, Ren D, Bakr O, Singh N, Kandemir U, Marmor MT, Morshed S (2016) Reduction of radiation exposure from c-arm fluoroscopy during orthopaedic trauma operations with introduction of real-time dosimetry. J Orthop Trauma 3(2):e53–e58
Bier B, Aschoff K, Syben C, Unberath M, Levenston M, Gold G, Fahrig R, Maier A (2018) Detecting anatomical landmarks for motion estimation in weight-bearing imaging of knees. In: International workshop on machine learning for medical image reconstruction. Springer, New York, pp 83–90
Bier B, Unberath M, Zaech JN, Fotouhi J, Armand M, Osgood G, Navab N, Maier A (2018) X-ray-transform invariant anatomical landmark detection for pelvic trauma surgery. In: International conference on medical image computing and computer-assisted intervention. Springer, New York, pp 55–63
Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward S, Miller J, Pieper S, Kikinis R (2012) 3d slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging 30(9):1323–1341
Ghesu FC, Georgescu B, Mansi T, Neumann D, Hornegger J, Comaniciu D (2016) An artificial agent for anatomical landmark detection in medical images. In: MICCAI. Springer, New York, pp 229–237
Ghesu FC, Georgescu B, Zheng Y, Grbic S, Maier A, Hornegger J, Comaniciu D (2017) Multi-scale deep reinforcement learning for real-time 3d-landmark detection in ct scans. IEEE Trans Pattern Anal Mach Intell 41:176–189
Härtl R, Lam KS, Wang J, Korge A, Audigé FKL (2013) Worldwide survey on the use of navigation in spine surgery. World Neurosurg 379(1):162–172
Hartley RI, Zisserman A (2004) Multiple view geometry in computer vision. Cambridge University Press, Cambridge. ISBN 0521540518
Heimann T, Meinzer HP (2009) Statistical shape models for 3d medical image segmentation: a review. Med Image Anal 13(4):543–563
Hou B, Alansary A, McDonagh S, Davidson A, Rutherford M, Hajnal JV, Rueckert D, Glocker B, Kainz B (2017) Predicting slice-to-volume transformation in presence of arbitrary subject motion. In: MICCAI. Springer, New York, pp 296–304
Johnson HJ, Christensen GE (2002) Consistent landmark and intensity-based image registration. IEEE Trans Med Imaging 21(5):450–461
Khurana B, Sheehan SE, Sodickson AD, Weaver MJ (2014) Pelvic ring fractures: what the orthopedic surgeon wants to know. Radiographics 34(5):1317–1333
Litjens G, Kooi T, Bejnordi BE, Setio AAA, Ciompi F, Ghafoorian M, van der Laak JA, Van Ginneken B, Sánchez CI (2017) A survey on deep learning in medical image analysis. Med Image Anal 42:60–88
Liu D, Zhou KS, Bernhardt D, Comaniciu D (2010) Search strategies for multiple landmark detection by submodular maximization. In: 2010 IEEE conference on computer vision and pattern recognition (CVPR), IEEE, pp 2831–2838
Mader AO, von Berg J, Fabritz A, Lorenz C, Meyer C (2018) Localization and labeling of posterior ribs in chest radiographs using a CRF-regularized FCN with local refinement. In: International conference on medical image computing and computer-assisted intervention. Springer, New York, pp 562–570
Markelj P, Tomaževič D, Likar B, Pernuš F (2012) A review of 3d/2d registration methods for image-guided interventions. Med Image Anal 16(3):642–661
Payer C, Štern D, Bischof H, Urschler M (2016) Regressing heatmaps for multiple landmark localization using CNNS. In: International conference on medical image computing and computer-assisted intervention. Springer, New York, pp 230–238
Pouch AM, Yushkevich PA, Jackson BM, Jassar AS, Vergnat M, Gorman JH, Gorman RC, Sehgal CM (2012) Development of a semi-automated method for mitral valve modeling with medial axis representation using 3d ultrasound. Med Phys 39(2):933–950
Roth H, Lu L, Seff A, Cherry KM, Hoffman J, Wang S, Summers RM (2015) A new 2.5 D representation for lymph node detection in CT. Cancer Imaging Arch. https://doi.org/10.7937/K9/TCIA.2015.AQIIDCNM
Sa R, Owens W, Wiegand R, Studin M, Capoferri D, Barooha K, Greaux A, Rattray R, Hutton A, Cintineo J, Chaudhary V (2017) Intervertebral disc detection in x-ray images using faster r-cnn. In: 2017 39th annual international conference of the IEEE engineering in medicine and biology society (EMBC), IEEE, pp 564–567
Starr R, Jones A, Reinert C, Borer D (2001) Preliminary results and complications following limited open reduction and percutaneous screw fixation of displaced fractures of the acetabulum. Injury 32:SA45–SA50
Štern D, Ebner T, Urschler M (2016) From local to global random regression forests: exploring anatomical landmark localization. In: International conference on medical image computing and computer-assisted intervention. Springer, New York, pp 221–229
Stöckle U, Schaser K, König B (2007) Image guidance in pelvic and acetabular surgery-expectations, success and limitations. Injury 38(4):450–462
Tucker E, Fotouhi J, Unberath M, Lee SC, Fuerst B, Johnson A, Armand M,Osgood GM, Navab N (2018) Towards clinical translation of augmented orthopedic surgery: from pre-op CT to intra-op x-ray via RGBD sensing. In: Medical imaging 2018: imaging informatics for healthcare, research, and applications, vol 10579. International Society for Optics and Photonics, p 105790J
Unberath M, Zaech JN, Lee SC, Bier B, Fotouhi J, Armand M, Navab N (2018) Deepdrr–a catalyst for machine learning in fluoroscopy-guided procedures. In: International conference on medical image computing and computer-assisted intervention. Springer, New York
Urschler M, Ebner T, Štern D (2018) Integrating geometric configuration and appearance information into a unified framework for anatomical landmark localization. Med Image Anal 43:23–36
Wang CW, Huang CT, Hsieh MC, Li CH, Chang SW, Li WC, Vandaele R, Marée R, Jodogne S, Geurts P, Chen C, Zhen G, Chu C, Mirzaalian H, Vrtovec T, Ibragimov B (2015) Evaluation and comparison of anatomical landmark detection methods for cephalometric x-ray images: a grand challenge. IEEE Trans Med Imaging 34(9):1890–1900
Wei SE, Ramakrishna V, Kanade T, Sheikh Y (2016) Convolutional pose machines. In: CVPR, pp 4724–4732
Xie W, Franke J, Chen C, Grützner PA, Schumann S, Nolte LP, Zheng G (2015) A complete-pelvis segmentation framework for image-free total hip arthroplasty (tha): methodology and clinical study. Int J Med Robot Comput Assist Surg 11(2):166–180
Zheng Y, Barbu A, Georgescu B, Scheuering M, Comaniciu D (2008) Four-chamber heart modeling and automatic segmentation for 3-d cardiac ct volumes using marginal space learning and steerable features. IEEE Trans Med Imaging 27(11):1668–1681
Acknowledgements
We gratefully acknowledge the support of NIH/NIBIB R01 EB023939, R21 EB020113, R01 EB016703, R01 EB0223939, and the NVIDIA Corporation with the donation of the GPUs used for this research. Further, the authors acknowledge Funding support from NIH 5R01AR065248-03.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bier, B., Goldmann, F., Zaech, JN. et al. Learning to detect anatomical landmarks of the pelvis in X-rays from arbitrary views. Int J CARS 14, 1463–1473 (2019). https://doi.org/10.1007/s11548-019-01975-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-019-01975-5