Abstract
Despite FDA suspension of Elan’s AN-1792 amyloid beta (Aβ) vaccine in phase IIb clinical trials, the implications of this study are the guiding principles for contemporary anti-Aβ immunotherapy against Alzheimer’s disease (AD). AN-1792 showed promising results with regards to Aβ clearance and cognitive function improvement, but also exhibited an increased risk of Th1 mediated meningoencephalitis. As such, vaccine development has continued with an emphasis on eliciting a notable anti-Aβ antibody titer, while avoiding the unwanted Th1 pro-inflammatory response. Previously, we published the first report of an Aβ sensitized dendritic cell vaccine as a therapeutic treatment for AD in BALB/c mice. Our vaccine elicited an anti-Aβ titer, with indications that a Th1 response was not present. This study is the first to investigate the efficacy and safety of our dendritic cell vaccine for the prevention of AD in transgenic mouse models (PDAPP) for AD. We also used Immunohistochemistry to characterize the involvement of LXR, ABCA1, and CD45 in order to gain insight into the potential mechanisms through which this vaccine may provide benefit. The results indicate that (1) the use of mutant Aβ1-42 sensitized dendritic cell vaccine results in durable antibody production, (2) the vaccine provides significant benefits with regards to cognitive function without the global (Th1) inflammation seen in prior Aβ vaccines, (3) histological studies showed an overall decrease in Aβ burden, with an increase in LXR, ABCA1, and CD45, and (4) the beneficial results of our DC vaccine may be due to the LXR/ABCA1 pathway. In the future, mutant Aβ sensitized dendritic cell vaccines could be an efficacious and safe method for the prevention or treatment of AD that circumvents problems associated with traditional anti-Aβ vaccines.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) is a devastating neurodegenerative disease affecting more than 26 million people worldwide. Contemporary treatments for AD simply modulate its symptoms, but no cure or prevention has yet been found (Van Norden et al. 2009; Götz et al. 2011; Saxena 2011). As aggregates of beta-amyloid (Aβ) are the major constituents of the senile plaques found in AD patients, Aβ has been considered a major etiological factor of the neuronal loss and cognitive decline seen in AD patients (Reitz et al. (2012); Octave 1995; Pillay et al. 2004). Thus, many groups have considered Aβ immunotherapy as a therapeutic treatment for AD. In 2000, an Aβ1-42 vaccine effectively removed amyloid plaques in the brains of APP transgenic (Tg) mice; corresponding behavioral improvements were also observed (Morgan et al. 2000). Soon thereafter, an Aβ vaccine (Elan’s AN-1792) with QS21 as an adjuvant entered clinical trials, but the study was suspended in phase IIb clinical trials due to the occurrence of brain encephalitis in 6 % of patients. However, long term follow-up studies did identify efficacy of the vaccine, as vaccinated patients showed a slower rate of cognitive decline and better disability assessment for dementia scores in comparison to control patients 4 and 10 years later (Vellas et al. 2009; Bayer et al. 2005).
Since then, there has been increased focus on the role of inflammation in AD (Akiyama et al. 2000; Tuppo and Arias 2005). Some reports have linked the QS21 adjuvant to initiation of a Th1 pro-inflammatory response, causing the meningoencephalitis observed in the AN-1792 trial (Mathews and Nixon 2003; Birmingham and Frantz 2002). Ever since, vaccine development has continued with an emphasis on avoiding the unwanted Th1 pro-inflammatory response while promoting the Th2 anti-inflammatory response. The Aβ vaccines currently in clinical trials use the B-cell epitope of the Aβ peptide as opposed to the T-cell epitope to avoid Th1 inflammation (Pfizer and JANSSEN Alzheimer Immunotherapy Research & Development 2007 - [cited] 2011 Aug 30; Merck 2007; Novartus 2011).
Recently, our lab has focused on the use of antigen-sensitized dendritic cells (DCs) for AD vaccine development. Antigen sensitized DCs have resulted in vaccines with progression into clinical trials for many diseases, and have circumvented vaccine related inflammation issues in vaccine development for other diseases (Satthaporn and Eremin 2001; Loveland et al. 2006; Cohen et al. 2005; Gajewski et al. 2001; Mittendorf et al. 2006). Our previous studies on DC vaccines in BALB/c mice and unpublished data on 2x-tg APP/PS1 mice show DCs sensitized with mutated Aβ peptides produce an antigenic response, with corresponding behavioral improvements (Cao et al. 2008). This study uses PDAPP transgenic mice, which overexpresses human APP, to test the efficacy of this vaccine for the prevention of AD (Games et al. 1995). Additionally, we focus on nuclear hormone liver X receptor (LXR), ABCA1, and CD45 in order to gain insights into the potential mechanism of action through which this treatment works. The aims of this study are to: (1) investigate the preventative usefulness of this vaccine by observation of antibody titer and/or Aβ load reduction, (2) determine cognitive benefit of vaccine, (3) determine if a Th1 inflammatory response was present, (4) determine pathways which may be activated through the use of DC vaccine.
Materials and methods
Animals
60 male C57BL/6 (C57) mice and 60 male PDAPP mice (4 months of age) were obtained from the Laboratory Animal Center of the PLA Academy of Military Science (Beijing, China) and equally divided into five groups (Table 1). The adjuvant and PDFM groups are the active vaccine groups, while the PWT, PBS, and DC group should not elicit an antibody titer. The PDFM, PWT, and DC groups all use vaccines that contain dendritic cells. All mice were handled with care and in accordance to the National Research Council’s guidelines for animals in research.
Vaccine preparation
DC preparation
DC preparation and sensitization followed the same protocol as previously described (Cao et al. 2008). In brief, bone marrow was removed from 7–11 week old female C57BL/6 mice and the femurs cleanly excised, all excess tissues were removed. Bones were merged in cold phosphate-buffered saline (PBS), washed with ethanol, and soaked in 1× PBS. The ends of each femur were cut from both sides, and bone marrow was flushed with 99 % RPMI + 1 % Antibiotic medium. Bone marrow was then gently re-suspended and passed through 70 μm sieves into a centrifuge tube. Following centrifugation for 10 min at 1100 rpm, the supernatant was removed and the pellets briefly vortexed. Red blood cells were lysed by incubation with 5 ml ACK (0.15 M NH4Cl, 1 mM KHCO3, 0.1 mM EDTA, pH 7.3 at room temperature) for 30 s while shaking, and lysis was stopped by the addition of 45 ml HBSS. After centrifugation at 1,100 rpm (10°C for 10 min), cells were suspended at 1 × 106 cells/ml in 99 % RPMI (+10 %-FBS) and 1 % Antibiotic. 10 ng/ml GM-CSF and 10 ng/ml IL-4 (BD-Pharmgen, San Jose CA) were added to the media and cells were cultured in 6-well plates (3 ml/well).
Peptide preparation
Wild Type Aβ1-42 (PWT) and the Aβ with the Dutch and Flemish mutations (PDFM) were synthesized by Biomer Tech (CA, USA) and used to sensitize DCs as follows. Peptide sequences are listed in Table 2.
Sensitized DC vaccine preparation
On the second day of DC culturing, the medium was completely aspirated and 3 ml of fresh DC culture medium was added. The cells were allowed to grow in a CO2 incubator (5 % CO2), and on day 4 of culturing, the cells were treated as follows: 1 ml/well old medium was aspirated and replaced by 1 ml/well fresh DC culture medium containing 60 μg/ml peptide (diluted to a final concentration 20 μg/ml). On the 8th day, DCs were harvested, washed twice, and the concentration adjusted to 5 × 106 cells/ml.
DC vaccine administration
For C57 mice: a single injection of 1 × 106 cells in 0.2 ml 1× PBS was administered via intraperitoneal (IP) injection. For PDAPP mice: 1 × 106 cells in 0.2 ml 1× PBS was administered by IP injection every 2 weeks; five total injections were administered (days 0, 14, 28, 42, and 56,). DC percentage of injected cells was <90 %.
Vaccine with adjuvant preparation and administration
Wild Type Aβ1-42 peptide was freshly prepared at 2 mg/mL from lyophilized powder as follows. 2 mg of the peptide was added to 0.9 mL deionized water and vortexed, 100 μl 10 x PBS (pH 7.5) was added. The resulting mixture was vortexed and incubated at 37°C overnight. The peptide was used (100 μg/injection) with a complete Freund adjuvant in a 1:1 (v/v) mixture as a vaccine. The vaccine with adjuvant was administered subcutaneously to the adjuvant group at the same time the DC vaccine was administered to the DC vaccine groups.
Sample collection and flow cytometry
For C57 mice: blood was collected in EDTA tubes by submandibular phlebotomy pre-immunization, and then weekly for 35 days (collection on days 0, 7, 14, 21, 28, and 35). C57 mice were euthanized after day 35. For PDAPP mice: blood samples were collected in EDTA tubes by submandibular phlebotomy bleeding pre-immunization, ten days after each vaccination, and post euthanasia (collection on days 0, 10, 24, 38, 52, 66, and 80). For each sample, plasma was isolated by centrifugation at 1,000 × g for 3 min, and transferred into screw-capped tubes and stored at −80°C. Flow cytometry was performed with fluorescent dye conjugated antibodies (i.e. CD11c, CD80, CD86, and MHC II, all antibodies from eBioscience, CA USA) to determine immunological effect of DC vaccine. All plasma samples were analyzed for anti-Aβ antibody levels and cytokine expression profiles.
Aβ antibody titer determination
An ELISA method was used to determine antibody levels in plasma samples using Aβ1-42 peptide as the binding antigen. 96-well plates were coated with 50 μl Aβ 1–42 in CBC buffer at 10 μg/ml, and a CBC plate was set up to determine background binding. Then, both Aβ and CBC plates were incubated at 4 °C overnight. After 5 washes with a wash buffer, plates were subject to a blocking step with 180 μl blocking buffer (1 × PBS containing 1.5 % BSA) for 45 min, then washed an additional 5 times with wash buffer. Samples diluted with blocking buffer were added to both Aβ plates and CBC-plates, with two-fold serial dilutions starting at 1:200, and incubated at 37 °C for 1 h., followed by 12 washes with wash buffer. HRP-conjugated anti-mouse IgG was loaded into each well at 1:5000 dilution with dilution buffer, incubated for 1 h. at 37 °C, then washed 12 times. TMB substrate was dissolved in PCB buffer and 100 μl was added into each well. The colorimetric reaction was stopped with 25 μl 2 N H2SO4. Plates were read at 450 nm/620 nm with a BioTek Synergy Reader. Samples having readings three times higher than controls were considered positive; the highest dilution was considered the endpoint titer.
Cytokine expression detection
A Luminex Liquidchip system (Panomics) following each step of the Liquidchip protocol as previously described to measure levels of G-CSF, IL-12, IL-17, IL-1α, IL-6, IFN-γ and TNF-α in serum (Cao et al. 2008).
Behavioral testing
One day before behavioral testing, all mice were allowed a swimming acclimatization session to reduce stress during behavioral testing. All mice were handled gently and with care to avoid unnecessary stress on the mice. On day 69, (i.e., at 6.5 months of age), mice began behavioral testing. MWM testing was preformed to acquire the mouse’s learning and memory ability. The MWM system was a small pool (diameter: 90 cm, depth: 50 cm) divided into four quadrants. An escape platform 10 cm in diameter was located in the 3rd quadrant 1.5 cm below the surface of the water and 30 cm from the edge of the pool. The water was made opaque and kept between 25-27°C.
First, the mice were trained twice daily according to the following protocol: the mice were gently lowered into the water (back first and facing the wall) in quadrant 1, 2, or 4. The mice were placed in the water in the middle of the chosen quadrant, which was randomly selected. Once the mice in the water, a CCD camera connected to a computer recorded each mouse’s swim path. Escape latency was recorded as the time from being put into the water to climbing the escape platform. If a mouse could not find the platform in 120 s, the escape latency was considered 120 s. The mouse must navigate to and stay on the platform for 15 s to complete a trail. After local navigation training for 11 days, space searching trials began on day 80. The platform was removed from the pool and the mouse put into the water (back first and facing the wall) in the middle of the 2nd quadrant. Number of crossing, location of platform and time of stay in the 3rd quadrant were recorded. The mice were euthanized after behavioral testing.
Histological studies
Mice were anesthetized with chloral hydrate (0.01 ml/g by i.p. injection) and perfused through the left ventricle with 0.9 % saline until the liver color changed from red to white. They were then perfused with 4 % paraformaldehyde-PBS solution for 2 min, and brain tissue was harvested as per established protocol (Scholtzova et al. 2009). The hemisphere was divided into three parts coronally, each approximately one third of the total brain and immersion-fixed in periodate-lysine-paraformaldehyde (PLP). After fixation, the brains were embedded in paraffin and stored until sectioning. Immunohistochemistry was performed on 4-μm paraffin sections. After a mouse IgG blocking step, serial coronal brain sections were stained with: (1) Beta-Amyloid mouse monoclonal antibody 6 F/3D (1:50, Vector), (2) mouse monoclonal [PPZ0412] to LXR alpha -ChIP Grade ab41902(1:100, Abcam), (3) anti-rabbit ABCA1 antibody (1:200, Novus), (4) Rat antimouse CD45 MCA 1031 G .(1:50, AbD Serotec). Immunostaining procedure is previously described (Postupna et al. 2011).
Quantitative image analysis
The stained tissue sections were scanned using a light microscope (Olympus BX41,Olymus Optical, Japan) at 400x magnification and images were captured with a digital camera. The specific brain region in question (hippocampus or cortex) was manually outlined and the total pixel area occupied by the structure was determined. A monochromatic based threshold was used for identification of immunofluorescencing areas. In the five tissue sections analyzed per animal, the CA1, CA2, CA3, DG, Rad of the hippocampus and the cortex on coronal plane sections were measured. Pixel intensities (gray level) ranged from 0 (densest-stained pattern; i.e., black) to 255 (lightest-stained pattern; i.e., white). An individual blinded to the experimental condition of the study performed all measurements.
Statistical analysis
All statistical analysis was performed using the SPSS software (SPSS 10.0.1). Data are presented as mean ± SEM. Data were analyzed using analysis of variance (ANOVA), as well as one-way ANOVA. Comparison of gray levels between each parametric groups was done using the unpaired two-tailed Students t test. P values of 0.05 or less were considered to be statistically significant.
Results
Immune system is activated by DCs vaccine
Average counts of CD11c, CD56, MHCII, and CD80-positive cells were significantly increased in the C57 PDFM group when compared to the DC only group. Cell counts were also increased in the PDFM group compared to the PWT group (P <0.005) (Figure 1). Immune cell counts increased because activated dendritic cells (those sensitized with mutant peptide) initiated an immune response, leading to activation of immune pathways. The in vitro maturation of DC’s provides the environment necessary for a successful interaction between APC cells and helper T cells.
PDFM vaccine can induce long lasting antibody response in both tg and non-tg mice
In C57 mice of the PDFM group, anti-Aβ antibody titer elevated significantly from day 14 onwards (Figure 2A). The elevated antibody titer retained to the 35th day after inoculation without reduction. The peak antibody titer level was 2,425.17, which was lower than vaccine with adjuvant (peak value 3,200). In C57 mice in the adjuvant group, the antibody titer increased significantly from day 7 to 21 post inoculation, at which point the titer of adjuvant group was significantly higher than that of the PDFM group (Students T-Test, P < .05). However, at 28 days there was no difference between groups, and at 35 days after inoculation titer of adjuvant group was lower than PDFM group significantly (Students T-Test, P < .05) Antibody titer levels of the three other group (DC, PBS and PWT stimulated DC) were not elevated at all.
When PDAPP-tg mice were vaccinated with the PDFM DC vaccine, anti-Aβ antibody titer increased significantly 24 days after inoculation. The elevated antibody titer remained until 66 days after inoculation without reduction. At the end of our experiment (80 days after inoculation), endpoint titer of anti-Aβ antibody in this group was 200. The peak of geographic mean antibody titer was 818.18, which was lower than vaccine with adjuvant (the peak value was 1,128.9). In PDAPP mice of the adjuvant group, antibody titer elevated significantly from day 10 after inoculation and fell again 40 days after inoculation. At the end of our experiment, titer of antibody in this group was 400. From days 10 to 66 days after inoculation, titer of adjuvant group was higher than PDFM group significantly (Students T-Test, P < .05). Beyond that point, there was no difference between these two groups. Titer of antibody of the other two groups (DC, PBS) had no elevation at all. The results are shown in Figure 2B.
Sensitized dendritic cells can process and present antigens via the traditional antigen presentation pathways, resulting in antibody production when a foreign antigen is present. In this case, the PDFM group elicited an antibody response because the Aβ peptide used contained two point-mutations in the Aβ T-cell epitope. As the PWT (wild type Aβ sensitized DC) group contained only a self-antigen, no antibody titer was elicited. The wild-type Aβ peptide can only elicit an antibody response when delivered with an adjuvant through subcutaneous injection, because adjuvants have the ability to prime the immune system and antigen presentation in the subcutaneous region differs from that in the blood.
DC vaccination correlates with cognitive benefits in PDAPP mice
Escape latency of vaccine therapy groups was significantly shorter statistically than the negative control PBS group and DC only group (ANOVA, Fisher’s Exact Test, P < .05). Observing each curve of the four groups, escape latency shortened quickly in the first 5 days, but shortened more slowly after day 5 or 6. When the escape latency was compared day-by-day, a significant difference was found between vaccine therapy groups (PDFM or adjuvant group) and control groups (DC or PBS group). No significant difference was found between the two vaccine groups or the two control groups (ANOVA, Fisher’s Exact Test, P < .05). Results are shown in Figure 3. Furthermore, the number of platform location crossing and time in target quadrant in vaccine therapy groups were significantly more in the PDFM group than negative control PBS group and DC group statistically (ANOVA, Fisher’s Exact Test, P < .05). No significant difference was found between the two active vaccine therapy groups (PDFM and adjuvant). The results are shown in Figure 4. The PDFM vaccine was able to slow down PDAPP decline by lessening Aβ accumulation via antibody-mediated removal.
Cytokine expression profile demonstrated no Th1 inflammation in DCs vaccine
Levels of G-CSF, IL-17 and IL-6 elevated significantly after DC vaccine inoculation but IL-10, IL-12, IL-1-α, IFN-γ and TNF-α did not (Student’s T-Test, P > .05). The cytokine profile after DC vaccine inoculation was markedly different from that of vaccine with adjuvant, which had significantly elevated level of IL-6, IL-1α, IFN-γ and TNF-α. As the PDFM group consists of IL-4 cultured DCs, injection of these cells tends to enhance the anti-inflammatory (Th2) response and inhibit the inflammatory (Th1) cascade (Banchereau and Steinman 1998). Mice of the adjuvant group showed a Th1 specific response, in part initiated by presence of the adjuvant. The results are shown in Figure 5.
Cytokine expression profile. a G-CSF levels are increased most in the PDFM group. b IL-12 is increased in the DC Group but not substantially elsewhere. c IL-17 increases in the DC group and PDFM group. d IL-1α increases substantially in the adjuvant group, but not PDFM. e IL-6 increases in the adjuvant, DC, and PDFM groups. f IFN-γ increases substantially in the adjuvant group. g TNF-α increases significantly in the adjuvant group
DCs vaccine lower Aβ burden in the brain of PDAPP mice
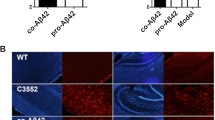
Both active vaccine groups (PDFM and adjuvant) resulted in less Aβ accumulation upon immunohistochemical staining. Mice in the PDFM group showed less accumulation in comparison with the DC only group in the hippocampus (Fig. 6K; ANOVA, p < 0.0001; 85.05 % decrease) and the cortex (Fig. 6L; ANOVA, p < 0.0001; 90.59 % decrease). PDAPP AD mice in the adjuvant group also showed a dramatic reduction in Aβ load in the hippocampus (Fig. 6E; ANOVA, p < 0.0001; 77.63 % decrease) and the cortex in comparison with the PBS control (Fig. 6F; ANOVA, P < 0.0001; 85.13 % decrease). There was a greater reduction of total amyloid burden in the PDFM mice than in the adjuvant group in the hippocampus (ANOVA, p = 0.002; 33.61 % decrease) and in the cortex (ANOVA, p = 0.043; 12.14 % decrease). There was no significant difference between the DC control and PBS controls (ANOVA, p = 0.66). The mechanisms through which antibodies lessen Aβ accumulation are discussed in detail later in the article. The results of CA3 of the hippocampus are shown in Fig. 6A, B, G, H and in the cortex in Fig. 6C, D, I, J.
Aβ accumulation. a Adjuvant group hippocampus b PBS group hippocampus c Adjuvant group cortex d PBS group cortex e Hippocampal comparative results f Cortical comparative results g PDFM group hippocampus h DC group hippocampus i PDFM group cortex j DC group cortex k Hippocampal comparative results l Cortical comparative results. Scale bar = 100 um (I,J). Scale bar = 50 um (A-D, G, H). Active vaccine groups showed a significant reduction in Aβ accumulation
LXR burden
As cholesterol is believed to be involved in the development of AD, we examined expression of LXR following vaccine treatment. LXR expression was highest in the PDFM group, followed by the DC group, with the adjuvant and PBS groups exhibiting the least expression (Fig. 7, A-D). The expression in the PDFM group was 29.30 % higher than that in the DC group (Fig. 7E, ANOVA, P = 0.0048). There was a 69.18 % and 61.46 % increase respectively when DC and adjuvant groups were compared with the PBS group (Fig. 7F, ANOVA, P<0.0001). Although there was a sharp difference in the expression, a comparison between the adjuvant and PBS groups did not yield statistically significant results (ANOVA, P = 0.19).
LXR quantification in the mouse brain. a PDFM group b DC group c Adjuvant group d PBS group e Comparative Results f Comparative Results. Scale bar = 500 um and Scale bar = 50 um. LXR expression was highest in the PDFM group, followed by the DC group, with adjuvant and PBS groups exhibiting the least expression
ABCA1 burden
Expression of ABCA1 was highest in the PDFM and DC groups, followed by the adjuvant group, with PBS showing the least expression (Fig. 8A-D). The level in the PDFM group was more than 2 times that of the DC group (Fig. 8E, ANOVA, p<0.0001). There was a clear increase in ABCA1 when comparing the DC and adjuvant groups to the PBS group (Fig. 8F, ANOVA, P<0.0001). There does not appear to be a statistical difference between the adjuvant and PBS groups (ANOVA, P = 0.86).
Expression and quantification of ABCA1 burden in mouse brains. a Adjuvant group b PDFM group c DC only group d PBS group e Comparative results f Comparative results. Hippocampal quantitative stereological analysis revealed a 2.12 times increase in PDFM compared with the DC group (E, ***p < 0.0001). ABCA1 increased significantly between the DC and adjuvant groups compared to the PBS or adjuvant group (F, ***P < 0.0001). Scale bar = 500 um and Scale bar = 50 um
CD45 burden
CD45 expression was highest in PDFM, then adjuvant, and finally the DC and PBS groups, with changes particularly evident in the hippocampal regions of the brain (Fig. 9A-D). The expression level within the hippocampus in the PDFM group was higher than that of the adjuvant group by 16.65 % (Fig. 9E, Student t test p = 0.034) while the DC and PBS groups showed a decrease of 74.48 % and 75.23 %, respectively (Fig 9F, ANOVA, P<0.0001). Similarly, CD45 expression in the PDFM group’s cortical areas was 15.70 % higher than those of the adjuvant group’s, (Fig. 9A. ANOVA p = 0.01), while the DC and PBS groups had a decrease compared to the adjuvant group of 78.46 % and 75.59 %, respectively (Fig. 9B. ANOVA, P < 0.0001). There was no significant difference between the two control groups in either the hippocampus (ANOVA, p = 0.85) or the cortex (ANOVA, p = 0.45). As CD45 is a marker for microglial activation, this supports our belief that the PDFM vaccine activated immune pathways in the CNS.
CD45 expression and quantification in mouse hippocampus. a Adjuvant group b PDFM group c DC group d PBS group e Hippocampal comparative results f Hippocampal comparative results g Cortical comparative results h Cortical comparative results. CD45 expression was highest in PDFM, then adjuvant, and finally the DC and PBS groups. Stereological CD45 burden analysis showed a significant reduction in adjuvant and PDFM compared with age-matched Tg control mice treated with PBS (E, F, adjuvant group vs. PDFM group, *p = 0.034). Scale bar = 500 um
Discussion
PDAPP mice express many of the neuropathological features of AD as early as 6 months of age, including synaptic loss, reduction of hippocampus size, reduction of fornix size, and reduction of corpus collosum size (Bryan et al. 2009). Additionally, in Morris Water Maze (MWM), open field, radial arm maze, operant bar pressing, and visual object recognition testing, PDAPP mice 6 months or older show significant memory impairments compared to age matched controls. These mice also show early extracellular Aβ deposition, and insoluble extracellular plaque formation shortly after 9 months of age (Schenk et al. 1999). Nonetheless, it is important to note that cognitive function deficits appear before the appearance of fully formed amyloid plaques (Dodart et al. 1999). Recent studies have focused on the oligomeric (soluble) form of Aβ, as opposed to the insoluble fibrils, as the neurotoxic agent in AD - implying that AD progression begins prior to plaque formation (Shankar et al. 2008; Kayed et al. 2003). As our study intended to investigate the preventative capability of our vaccine, we began PDAPP mice injections at 4 months of age, prior to the onset of significant cognitive decline. AD progression is underway during this period, as significant cognitive decline is expected by 6 months of age (Chen et al. 2000). At the time of behavioral testing (6.5 months of age), PDAPP are expected to show significant cognitive decline when compared to age matched control, thus testing the efficacy of this vaccine in prevention (or delay of onset) of AD.
A recent review by Tabira laid out conditions that must be met as AD vaccine development moves forward. To overcome the problems faced by past efforts, a new vaccine must avoid autoimmune encephalitis, be useful for disease prevention, modify disease course, and provide some sort of cognitive benefit (2010). Our vaccine has certain advantages over other AD vaccines in development, which are discussed below. Although both active and passive immunotherapy vaccines are in development, active immunization requires fewer treatments and provides a longer lasting antibody response at a lower cost. Moreover, studies have shown passive immunization in AD can result in vasogenic edema and brain microhemorrhaging in mouse models (Panza et al. 2011). Although active immunization does have drawbacks (ex. potential side effects: fever, swelling, etc.), these drawbacks are less probable with DC vaccines as antigen presentation is done ex vivo. This ex vivo antigen presentation also circumvents the need for direct Aβ injection, which can cross the blood brain barrier and act as a “seed” for enhanced Aβ fibril formation (Sigurdsson et al. 2002). Our vaccine avoids further aggression of Aβ oligomerization via introduction of this Aβ “seed.” Additionally, As DCs may be donated from the AD patient and cultured, the chances of graft-versus-host disease are eliminated (Smit et al. 1997).
Mosca et al., reported several DC vaccine cancer trials that demonstrated varying degrees of efficacy, but avoided unwanted inflammatory responses while being extremely well tolerated by patients (Mosca et al. 2007). The data shows that Th1 response was minimized in the PDFM group compared to the adjuvant group. IFN-γ, the most typical Th1 cytokine in sera, its was only observed in the adjuvant group, and took 50 days to fall back to normal levels. TNF-α, another typical Th1 cytokine, was only observed in the adjuvant group and sustained without reduction. Similar changes were found in IL-1α sera concentration curves. The cytokine profile of the DC vaccine groups showed no increase in Th1 activity. The obvious implication, that the adjuvant but not the production of anti-Aβ antibodies, played a role in Th1 activation, is an encouraging indicator that this vaccine may overcome the problems of the AN-1792 trials. Additionally, the concentration of sera IL-17 in the PDFM group elevated significantly in these studies and sustained until the end of the experiment. Similar phenomenon was found in the DC group, but it fell to normal level earlier. Elevation of IL-17 level is essential for breakthrough in immune tolerance or implied generation of autoimmune response, which needs to be characterized further. It is our belief that DC’s increase Th2 (anti-inflammatory) response, further opposing the immune response that caused adverse conditions in the AN-1792 trial. Presumably, treatment of the DC’s with IL-4 in culture prior to vaccine administration augmented the release of Th2 cytokines by the DCs. Regrettably, Ig isotyping was unable to be completed on these samples. However, past studies on this same vaccine in BALB/c mice and unpublished data on PS1/APP mice show preferential IgG1 production, indicating IL-4 mediated Th2 activation (Cao et al. 2009; Cao et al. 2008). Moreover, the literature shows that DC’s treated with IL-4 induce Th2 T-cell differentiation (Banchereau and Steinman 1998).
Vaccine development in the past has used different approaches to elicit a Th2 response, such as using mannan or lysine cores, however, our method of achieving a Th2 response is the most straightforward one. Additionally, the use of multiple copies of Aβ 1–6 (as some contemporary vaccines use) requires use of an adjuvant to develop a sufficient antibody titer. Use of an adjuvant can have adverse effects, as seen in human AN-1792 trials. As DCs themselves are able to act as a self adjuvant (Hart 1997), a separate adjuvant is not necessary. Above all, peptide sensitized DC vaccines have long lasting antigen-specific T-cell response that are typically not seen in traditional vaccines (Steinman 2001).
The PDFM sensitized vaccine in this study induces PDAPP-tg mice to produce enough antibody titer to signify a notable immune response, even 80 days after inoculation. More importantly, treatment with the PDFM vaccine correlated well to a decreased Aβ burden in immunohistochemical studies. Although only intracellular amyloid beta was observed, this result was expected as PDAPP mice develop extracellular plaques only at 9 months of age. However, the significant reduction of Aβ accumulation (85 % in cortex and 90 % in hippocampus) in the PDFM group compared to the control group provided enough protection to lead to significant increases in cognitive ability. Although the peak of antibody titer curve in the PDFM group was lower than that of the adjuvant group, the reduction of Aβ deposition was greater (85.05 % & 90.59 % clearance vs. 77.63 % & 95.13 %). Interestingly, though the PWT group does not elicit an antibody response, we observed a significant reduction of Aβ accumulation, implicating sensitized dendritic cells themselves may play a role in the reduction of Aβ accumulation through mechanisms separate from antibody mediated clearance.
The mechanism through which dendritic cell vaccines cause Aβ clearance is still not clear; however our study examined multiple potential targets through which this treatment may work. The most obvious mechanism is through antibody production, and four potential pathways have been hypothesized to play a role in antibody-mediated clearance of Aβ. A recent review by Morgan has laid out these mechanisms (2011). The first mechanism, based on conventional roles of antibodies to opsonize targets, suggests macrophage activity to explain Aβ clearance (Bard et al. 2000). However, the fact that only a small percentage of circulating antibodies make their way across the blood brain barrier, has led to proposal of a second proposed mechanism that describes a peripheral sink (DeMattos et al. 2001). This mechanism suggests that binding of antibodies to Aβ in the blood causes efflux of Aβ from the brain. The third mechanism suggests a conformational change that prevents oligomerization as a result of Aβ peptide and antibody interactions (Solomon et al. 1997). The final mechanism theorizes that Fc receptor (FcRn) mediated efflux of Ab-Ag complexes across blood brain barrier augment Aβ clearance (Deane et al. 2003). Viewing our results through the scope of these mechanisms, the lower antibody titer in the PDFM Vaccine should not have an appreciable effect on Aβ clearance. Studies have shown that a 1:1000 antibody to Aβ peptide ratio still results in clearance (NA and JJ 2010). Furthermore, studies have shown that excess antibody can reduce clearance, presumably due to the FcRn mediated mechanism (Karlnoski et al. 2008).
One other important observation in the cytokine profile of the PDFM that may provide mechanistic insight into the effect of DC Vaccines was the concentration levels of G-CSF. The concentration of G-CSF elevated significantly compared to the other three groups and was sustained through the duration of the experiment. G-CSF can mobilize activation of neutrophilic granulocytes and stimulate proliferation and differentiation of hematopoietic cells. Samchez-ramos et al. (Tsai et al. 2007) have reported G-CSF could be used as therapy for memory impairment in AD-tg mice. The mechanism is believed to be mobilization of hematopoietic stem cells followed by implantation in brain, facilitating neural regeneration. Elevation of G-CSF concentration in PDFM group is thought to enhance therapeutic effect.
To further understand the mechanisms through which the vaccine may function, we performed immunostaining to ascertain the expression levels of three possible targets: LXR, ABCA1, and CD45. Many groups have confirmed a correlation between cholesterol homeostasis and AD pathology (Simons et al. 2001). The ABCA1 staining patterns seen in our study are similar to those reported by Koldamova et al. (Koldamova et al. 2003). LXR is a regulatory nuclear receptor involved in the control of genes participating in removal of excess cholesterol through efflux, catabolism or decreased absorption. Transcriptional activation of ABCA1, a member of the ATP-binding cassette family of transporters that eliminates excess cholesterol from the cell, is controlled by the LXRα/β. Further support for this is shown by the marked increase in ABCA1 expression in neurons and glial cells after application of the Liver X Receptor agonist T0901317, resulting in an amelioration of AD pathology through brain inflammation and amyloid deposition (Lefterov et al. 2007). The enhanced therapeutic effect of our DC vaccine in Aβ clearance compared to the adjuvant vaccine may be due to the LXR/ABCA1 pathway, as both LXR and ABCA1 were significantly upregulated with the PDFM treatment but not with adjuvant treatment. Though the connection between a shift in the cholesterol pathways and DC vaccine immunization is not clear, the flow cytometry results indicate a general increase in immune cells in vaccinated mice. The activation of immune response initiated by activated dendritic cell inoculation may result in a shift in immune milieu in the CNS, thus changing the expression of cholesterol pathways. The specific mechanisms through which these changes occur need to be further investigated.
The “inflammation hypothesis of AD” implicates microglial activation and long-term inflammation in initiation of a proinflammatory cascade. The release of inflammatory cytokines is hypothesized to bring about degenerative changes in neurons and further microglial activation (Akiyama et al. 2000). Recent studies have shown that a CD45 deficiency brings about an increase in Aβ oligomers (Zhu et al. 2011), as CD45 is an marker for microglial activation (Stein et al. 2007). The increase in CD45 due to the PDFM treatment may play a role in immune mediated reduction of Aβ accumulation.
The results of the behavioral test support our hypothesis that the immune response generated by the DC Vaccine provides a level of protection, seemingly equal to the vaccine and adjuvant used in AN-1792 without the inflammatory response. Vaccinated PDAPP mice performed better on the cognitive function tests than their untreated counterparts, indicating the vaccine slowed AD pathology. This vaccine shows promising results in Aβ therapy for AD. Most other problems faced by this vaccine in treatment of AD are problems that will be faced by all Aβ directed therapies. It has been suggested that Aβ clearance may not correlate well with cognitive benefits. If this is found to be the case, Aβ therapy may be used in conjunction with another type of therapy. Furthermore, combined with an early biomarker and advanced diagnostic techniques, this vaccine can provide protective value before the onset of cognitive decline.
Conclusion
Our vaccine combines two desirable traits for an Alzheimer’s vaccine, the ability to produce a strong antibody response using the T-cell epitope and avoidance of a Th1 inflammatory reaction. This study shows the protective effect of DC vaccines functions through multiple pathways, including the LXR/ABCA1 pathway. Moreover, this vaccine may be used for both the prevention and treatment method of AD. Coupled with biomarkers for early identification of at risk individuals, this treatment may help lower the incidence of AD in the future.
References
Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T (2000) Inflammation and Alzheimer's disease. In: Neurobiol Aging, vol 21. vol 3. United States, pp 383–421
Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T (2000) Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med 6(8):916–919. doi:10.1038/78682
Bayer AJ, Bullock R, Jones RW, Wilkinson D, Paterson KR, Jenkins L, Millais SB, Donoghue S (2005) Evaluation of the safety and immunogenicity of synthetic Abeta42 (AN1792) in patients with AD. In: Neurology, vol 64. vol 1. United States, pp 94–101. doi:10.1212/01.wnl.0000148604.77591.67
Birmingham K, Frantz S (2002) Set back to Alzheimer vaccine studies. In: Nat Med, vol 8. vol 3. United States, pp 199–200. doi:10.1038/nm0302-199b
Bryan KJ, Lee H, Perry G, Smith MA, Casadesus G (2009) Transgenic mouse models of Alzheimer's disease: behavioral testing and considerations methods of behavior analysis in neuroscience. Taylor & Francis Group, LLC, Boca Raton
Cao C, Arendash GW, Dickson A, Mamcarz MB, Lin X, Ethell DW (2009) Abeta-specific Th2 cells provide cognitive and pathological benefits to Alzheimer's mice without infiltrating the CNS. Neurobiol Dis 34(1):63–70. doi:10.1016/j.nbd.2008.12.015
Cao C, Lin X, Zhang C, Wahi M, Wefes I, Arendash G, Potter H (2008) Mutant amyloid-beta-sensitized dendritic cells as Alzheimer's disease vaccine. Journal of Neuroimmunology 200(1–2):1–10
Cohen S, Haimovich J, Hollander N (2005) B-cell lymphoma and myeloma protection induced by idiotype vaccination with dendritic cells is mediated entirely by T cells in mice. In: J Immunother, vol 28. vol 5. United States, pp 461–466
Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B (2003) RAGE mediates amyloid-beta peptide transport across the blood–brain barrier and accumulation in brain. In: Nat Med, vol 9. vol 7. United States, pp 907–913. doi:10.1038/nm890
DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM (2001) Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer's disease. In: Proc Natl Acad Sci U S A, vol 98. vol 15. United States, pp 8850–8855. doi:10.1073/pnas.151261398
Dodart JC, Meziane H, Mathis C, Bales KR, Paul SM, Ungerer A (1999) Behavioral disturbances in transgenic mice overexpressing the V717F beta-amyloid precursor protein. Behav Neurosci 113(5):982–990
Chen et al (2000) A learning deficit related to age and beta-amyloid plaques in a mouse model of. Nature 408(6815):975–979
Gajewski TF, Fallarino F, Ashikari A, Sherman M (2001) Immunization of HLA-A2+ melanoma patients with MAGE-3 or MelanA peptide-pulsed autologous peripheral blood mononuclear cells plus recombinant human interleukin 12. Clin Cancer Res 7(3 Suppl):895s–901s
Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F et al (1995) Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature 373(6514):523–527. doi:10.1038/373523a0
Götz J, Eckert A, Matamales M, Ittner L, Liu X (2011) Modes of Aβ toxicity in Alzheimer’s disease. Cellular and Molecular Life Sciences:1–17. doi:10.1007/s00018-011-0750-2
Hart DN (1997) Dendritic cells: unique leukocyte populations which control the primary immune response. Blood 90(9):3245–3287
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392(6673):245–252
Karlnoski RA, Rosenthal A, Alamed J, Ronan V, Gordon MN, Gottschall PE, Grimm J, Pons J, Morgan D (2008) Deglycosylated anti-Abeta antibody dose–response effects on pathology and memory in APP transgenic mice. J Neuroimmune Pharmacol 3(3):187–197. doi:10.1007/s11481-008-9114-6
Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG (2003) Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. In: Science, vol 300. vol 5618. United States, pp 486–489. doi:10.1038/nm1782, 10.1126/science.1079469
Koldamova RP, Lefterov IM, Ikonomovic MD, Skoko J, Lefterov PI, Isanski BA, DeKosky ST, Lazo JS (2003) 22R-hydroxycholesterol and 9-cis-retinoic acid induce ATP-binding cassette transporter A1 expression and cholesterol efflux in brain cells and decrease amyloid beta secretion. J Biol Chem 278(15):13244–13256. doi:10.1074/jbc.M300044200
Lefterov I, Bookout A, Wang Z, Staufenbiel M, Mangelsdorf D, Koldamova R (2007) Expression profiling in APP23 mouse brain: inhibition of Abeta amyloidosis and inflammation in response to LXR agonist treatment. Mol Neurodegener 2:20
Loveland BE, Zhao A, White S, Gan H, Hamilton K, Xing PX, Pietersz GA, Apostolopoulos V, Vaughan H,Karanikas V, Kyriakou P, McKenzie IF, Mitchell PL (2006) Mannan-MUC1-pulsed dendritic cellimmunotherapy: a phase I trial in patients with adenocarcinoma. In: Clin Cancer Res, vol 12. vol 3 Pt 1. United States, pp 869–877. doi:10.1158/1078-0432.ccr-05-1574
Mathews PM, Nixon RA (2003) Setback for an Alzheimer's disease vaccine: lessons learned. Neurology 61(1):7–8
Merck (2007 - [cited] 2011 Aug 30) A study of V950 in people with Alzheimer Disease (V950-001). In: Clinicaltrials.gov [Internet]. http://clinicaltrials.gov/ct2/show/NCT00464334?term=V950&rank=1.
Mittendorf EA, Storrer CE, Foley RJ, Harris K, Jama Y, Shriver CD, Ponniah S, Peoples GE (2006) Evaluation of the HER2/neu-derived peptide GP2 for use in a peptide-based breast cancer vaccine trial. Cancer 106(11):2309–2317. doi:10.1002/cncr.21849
Morgan D (2011) Immunotherapy for Alzheimer's disease. J Intern Med 269(1):54–63. doi:10.1111/j.1365- 2796.2010.02315.x
Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW (2000) A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature 408(6815):982–985. doi:10.1038/35050116
Mosca PJ, Lyerly HK, Clay TM, Morse MA (2007) Dendritic cell vaccines. In: Front Biosci, vol 12. United States, pp 4050–4060
NA A, JJ, D M (2010) Direct observation of the kinetic mechanisms for Aß peptide aggregation: Towards elucidating Alzheimer plaque dissolution. vol 6:S247. Alzheimers Dement.
Novartus (2011) To Investigate the Safety and Tolerability of Repeated Subcutaneous Injections of CAD106 in Alzheimer's Patients. Clinicaltrials.gov [Internet], Bethesda (MD): National Library of Medicine (US)
Octave JN (1995) The amyloid peptide and its precursor in Alzheimer’s disease. Rev Neurosci 6(4):287–316
Panza F, Frisardi V, Imbimbo BP, Seripa D, Solfrizzi V, Pilotto A (2011) Monoclonal antibodies against beta-amyloid (Abeta) for the treatment of Alzheimer's disease: the Abeta target at a crossroads. Expert Opin Biol Ther 11(6):679–686. doi:10.1517/14712598.2011.579099
Pfizer, JANSSEN Alzheimer Immunotherapy Research & Development L (2007 - [cited] 2011 Aug 30) Study Evaluating ACC-001 In Subjects With Mild To Moderate Alzheimer's Disease. In: ClinicalTrials.gov [Internet]. http://clinicaltrials.gov/ct2/show/NCT00498602?intr=%22ACC-001%22&rank=5.
Pillay NS, Kellaway LA, Kotwal GJ (2004) Molecular mechanisms, emerging etiological insights and models to test potential therapeutic interventions in Alzheimer’s disease. Curr Alzheimer Res 1(4):295–306
Postupna N, Rose SE, Bird TD, Gonzalez-Cuyar LF, Sonnen JA, Larson EB, Keene CD, Montine TJ (2011) Novel antibody capture assay for paraffin-embedded tissue detects wide-ranging amyloid beta and paired helical filament-tau accumulation in cognitively normal older adults. Brain Pathol. doi:10.1111/j.1750-3639.2011.00542.x
Reitz C, Brayne C, Mayeux R (2012) Epidemiology of Alzheimer disease.
Satthaporn S, Eremin O (2001) Dendritic cells (II): Role and therapeutic implications in cancer. J R Coll Surg Edinb 46(3):159–167
Saxena U (2011) Bioenergetics breakdown in Alzheimer's disease: targets for new therapies. Int J Physiol Pathophysiol Pharmacol 3(2):133–139
Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P (1999) Immunization with amyloid- beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400(6740):173–177. doi:10.1038/22124
Scholtzova H, Kascsak RJ, Bates KA, Boutajangout A, Kerr DJ, Meeker HC, Mehta PD, Spinner DS, Wisniewski T (2009) Induction of toll-like receptor 9 signaling as a method for ameliorating Alzheimer's disease-related pathology. In: J Neurosci, vol 29. vol 6. United States, pp 1846–1854. doi:10.1523/jneurosci.5715-08.2009
Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ (2008) Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med 14(8):837–842
Sigurdsson EM, Wisniewski T, Frangione B (2002) A safer vaccine for Alzheimer's disease? In: Neurobiol Aging, vol 23. vol 6. United States, pp 1001–1008
Simons M, Keller P, Dichgans J, Schulz JB (2001) Cholesterol and Alzheimer's disease: is there a link? Neurology 57(6):1089–1093
Smit WM, Rijnbeek M, van Bergen CA, de Paus RA, Vervenne HA, van de Keur M, Willemze R, Falkenburg JH (1997) Generation of dendritic cells expressing bcr-abl from CD34-positive chronic myeloid leukemia precursor cells. In: Hum Immunol, vol 53. vol 2. United States, pp 216–223. doi:10.1016/s0198-8859(96)00285-6
Solomon B, Koppel R, Frankel D, Hanan-Aharon E (1997) Disaggregation of Alzheimer beta-amyloid by site- directed mAb. Proc Natl Acad Sci U S A 94(8):4109–4112
Stein VM, Baumgartner W, Schroder S, Zurbriggen A, Vandevelde M, Tipold A, Zelcer N, Khanlou N, Clare R, Jiang Q, Reed-Geaghan EG, Landreth GE, Vinters HV, Tontonoz P (2007) Differential expression of CD45 on canine microglial cells attenuation of neuroinflammation and Alzheimer's disease pathology by liver x receptors. In: J Vet Med A Physiol Pathol Clin Med, vol 54. vol 6. Germany, United States, pp 314–320. doi:10.1172/jci3190910.1111/j.1439-0442.2007.00926.x
Steinman RM (2001) Dendritic cells and the control of immunity: enhancing the efficiency of antigen presentation. Mt Sinai J Med 68(3):160–166
Tabira T (2010) Immunization therapy for Alzheimer disease: a comprehensive review of active immunization strategies. In: Tohoku J Exp Med, vol 220. vol 2. Japan, pp 95–106
Tsai KJ, Tsai YC, Shen CK (2007) G-CSF rescues the memory impairment of animal models of Alzheimer's disease. In: J Exp Med, vol 204. vol 6. United States, pp 1273–1280. doi:10.1084/jem.20062481
Tuppo EE, Arias HR (2005) The role of inflammation in Alzheimer's disease. In: Int J Biochem Cell Biol, vol 37. vol 2. England, pp 289–305. doi:10.1016/j.biocel.2004.07.009
Van Norden AGW, van Dijk EJ, de Laat KF, Scheltens P, OldeRikkert MGM, de Leeuw FE, Vellas B, Black R, Thal LJ, Fox NC, Daniels M, McLennan G, Tompkins C, Leibman C, Pomfret M, Grundman M (2009) Long-term follow-up of patients immunized with AN1792: reduced functional decline in antibody responders. Curr Alzheimer Res 6(2):144–151
Zhu Y, Hou H, Rezai-Zadeh K, Giunta B, Ruscin A, Gemma C, Jin J, Dragicevic N, Bradshaw P, Rasool S, Glabe CG, Ehrhart J, Bickford P, Mori T, Obregon D, Town T, Tan J (2011) CD45 deficiency drives amyloid- beta peptide oligomers and neuronal loss in Alzheimer's disease mice. J Neurosci 31(4):1355–1365. doi:10.1523/jneurosci.3268-10.2011
Acknowledgements
This research was supported by funds from key project of Tianjin Municipal Science and Technology Commission (09JCZDJC20200), as well as by the funds from key project of Tianjin Public Health Bureau (06KG09).
Conflicts of Interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Zhongqiu Luo and Jialin Li contribute equally to the work
Rights and permissions
About this article
Cite this article
Luo, Z., Li, J., Nabar, N.R. et al. Efficacy of a Therapeutic Vaccine Using Mutated β-amyloid Sensitized Dendritic Cells in Alzheimer’s Mice. J Neuroimmune Pharmacol 7, 640–655 (2012). https://doi.org/10.1007/s11481-012-9371-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-012-9371-2