Abstract
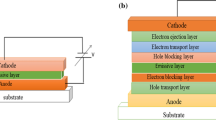
The aggregation-induced emission (AIE) phenomenon provides a new direction for the development of organic light-emitting devices. Here, we present a new class of emitters based on 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY), functionalized at different positions with tetraphenylethylene (TPE), which is one of the most famous AIE luminogens. Thanks to this modification, we were able to tune the photoluminescence of the BODIPY moiety from the green to the near-infrared (NIR) spectral range and achieve PL efficiencies of ~50% in the solid state. Remarkably, we observed an enhancement of the AIE and up to ~100% photoluminescence efficiencies by blending the TPE-substituted BODIPY fluorophores with a poly[(9,9-di-n-octylfluorene-2,7-diyl)-alt-(benzo[2,1,3]thiadiazol-4,7-diyl)] (F8BT) matrix. By incorporating these blends in organic light-emitting diodes (OLEDs), we obtained electroluminescence peaked in the range 650–700 nm with up to 1.8% external quantum efficiency and ~2 mW/cm2 radiance, a remarkable result for red/NIR emitting and solution-processed OLEDs.
Similar content being viewed by others
References
Boens N, Leen V, Dehaen W. Chem Soc Rev, 2012, 41: 1130–1172
Zampetti A, Minotto A, Squeo BM, Gregoriou VG, Allard S, Scherf U, Chochos CL, Cacialli F. Sci Rep, 2017, 7: 1611
Murto P, Minotto A, Zampetti A, Xu X, Andersson MR, Cacialli F, Wang E. Adv Opt Mater, 2016, 4: 2068–2076
Marechal E. Prog Org Coatings, 1982, 10: 251–287
Ulrich G, Ziessel R, Harriman A. Angew Chem Int Ed, 2008, 47: 1184–1201
Loudet A, Burgess K. Chem Rev, 2007, 107: 4891–4932
Ziessel R, Ulrich G, Harriman A. New J Chem, 2007, 31: 496
Chen M, Li L, Nie H, Tong J, Yan L, Xu B, Sun JZ, Tian W, Zhao Z, Qin A, Tang BZ. Chem Sci, 2015, 6: 1932–1937
Dong W, Pina J, Pan Y, Preis E, Seixas de Melo JS, Scherf U. Polymer, 2015, 76: 173–181
Zhao Z, Chen S, Lam JWY, Wang Z, Lu P, Mahtab F, Sung HHY, Williams ID, Ma Y, Kwok HS, Tang BZ. J Mater Chem, 2011, 21: 7210
Zhao Z, Chen S, Lam JWY, Lu P, Zhong Y, Wong KS, Kwok HS, Tang BZ. Chem Commun, 2010, 46: 2221
Zhao Q, Zhang S, Liu Y, Mei J, Chen S, Lu P, Qin A, Ma Y, Sun JZ, Tang BZ. J Mater Chem, 2012, 22: 7387
Yuan WZ, Lu P, Chen S, Lam JWY, Wang Z, Liu Y, Kwok HS, Ma Y, Tang BZ. Adv Mater, 2010, 22: 2159–2163
Luo J, Xie Z, Lam JWY, Cheng L, Tang BZ, Chen H, Qiu C, Kwok HS, Zhan X, Liu Y, Zhu D. Chem Commun, 2001, 18: 1740–1741
Mei J, Hong Y, Lam JWY, Qin A, Tang Y, Tang BZ. Adv Mater, 2014, 26: 5429–5479
Gomez-Duran CFA, Hu R, Feng G, Li T, Bu F, Arseneault M, Liu B, Peña-Cabrera E, Tang BZ. ACS Appl Mater Interfaces, 2015, 7: 15168–15176
Hu R, Lager E, Aguilar-Aguilar A, Liu J, Lam JWY, Sung HHY, Williams ID, Zhong Y, Wong KS, Peña-Cabrera E, Tang BZ. J Phys Chem C, 2009, 113: 15845–15853
Baglan M, Ozturk S, Gür B, Meral K, Bozkaya U, Bozdemir OA, Atılgan S. RSC Adv, 2013, 3: 15866
Li Q, Qian Y. New J Chem, 2016, 40: 7095–7101
Li Z, Chen Y, Lv X, Fu WF. New J Chem, 2013, 37: 3755
Hu R, Gómez-Durán CFA, Lam JWY, Belmonte-Vázquez JL, Deng C, Chen S, Ye R, Peña-Cabrera E, Zhong Y, Wong KS, Tang BZ. Chem Commun, 2012, 48: 10099
Zhao Z, Chen B, Geng J, Chang Z, Aparicio-Ixta L, Nie H, Goh CC, Ng LG, Qin A, Ramos-Ortiz G, Liu B, Tang BZ. Part Part Syst Charact, 2014, 31: 481–491
Gao H, Gao Y, Wang C, Hu D, Xie Z, Liu L, Yang B, Ma Y. ACS Appl Mater Interfaces, 2018, 10: 14956–14965
Petrozza A, Brovelli S, Michels JJ, Anderson HL, Friend RH, Silva C, Cacialli F. Adv Mater, 2008, 20: 3218–3223
Tram K, Yan H, Jenkins HA, Vassiliev S, Bruce D. Dyes Pigments, 2009, 82: 392–395
Englman R, Jortner J. Mol Phys, 1970, 18: 145–164
Acknowledgements
This work was supported by the European Community’s Seventh Framework Programme (FP7/2007-2013) (607585). S.B. and S.A. thanks Stefan Türk from MPI, Anke Helfer and Sylwia Adamczyk from Bergische Universität Wuppertal for mass and AFM characterizations. S.B. and S.A. thank Dr. Michael Forster from BUW for the helpful discussions. S.B. thanks Prof. Ullrich Scherf and Bergische Universität Wuppertal for the financial support. F.C. is a Royal Society Wolfson Research Merit Award holder.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Baysec, S., Minotto, A., Klein, P. et al. Tetraphenylethylene-BODIPY aggregation-induced emission luminogens for near-infrared polymer light-emitting diodes. Sci. China Chem. 61, 932–939 (2018). https://doi.org/10.1007/s11426-018-9306-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-018-9306-2