Abstract
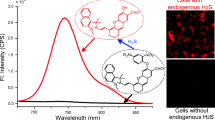
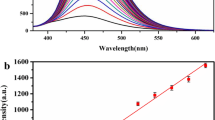
Hydrogen sulfide (H2S) has been found to be the third most important endogenous gaseous signaling molecule after nitric oxide (NO) and carbonic oxide (CO) and plays crucial roles in living organisms and biological systems. Here we use aggregation- induced emission (AIE) of a small organic molecule (TPE-indo) to detect H2S in both solution and living cells. TPE-indo can target mitochondria and aggregate to fluoresce, which can serve as a sensor for monitoring H2S in the mitochondria. We regulate the fluorescence of AIE molecules by tuning the viscosity of the solution to form TPE-indo nanoparticles, constructing a probe for H2S with good selectivity and high sensitivity. The nucleophilic addition of HS- to the TPE-indo is crucial for the rapid H2S detection. The imaging and analysis of H2S in mitochondria of living cells with the probe demonstrate potential biological applications.
Similar content being viewed by others
References
Li L, Moore P. Biochem Soc T, 2007, 35: 1138–1141
Lavu M, Bhushan S, Lefer DJ. Clin Sci, 2011, 120: 219–229
Pan LL, Liu XH, Gong QH, Zhu YZ. Amino Acids, 2011, 41: 205–215
Gong QH, Shi XR, Hong ZY, Pan LL, Liu XH, Zhu, YZ. J Alzheimers Dis, 2011, 24: 173–182
Whiteman M, Gooding KM, Whatmore JL, Ball CI, Mawson D, Skinner K, Tooke JE, Shore AC. Diabetologia, 2010, 53: 1722–1726
Abe K, Kimura H. J Neurosci, 1996, 16: 1066–1071
Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H. J Biochem, 2009, 146: 623–626
Kamoun P, Belardinelli MC, Chabli A, Lallouchi K, Chadefaux- Vekemans B. Am J Med Genet A, 2003, 116: 310–311
Yang W, Yang GD, Jia XM, Wu LY, Wang R. J Physiol, 2005, 569: 519–531
Yang GD, Wu LY, Jiang B, Yang W, Qi JS, Cao K, Meng QH, Mustafa AK, Mu WT, Zhang SM, Snyder SH, Wang R. Science, 2008, 322: 587–590
Fiorucci S, Antonelli E, Mencarelli A, Oriandi S, Renga B, Rizzo G, Distrutti E, Shah V, Morelli A. Hepatology, 2005, 42: 539–548
Eto K, Asada T, Arima K, Makifuchi T, Kimura H. Biochem Bioph Res Co, 2002, 293: 1485–1488
Kashfi K, Olson KR. Biochem Pharmacol, 2013, 85: 689–703
Kamoun P. Amino Acids, 2004, 26: 243–254
Mikami Y, Shibuya N, Kimura Y, Nagahara N, Ogasawara Y, Kimura H. Biochem J, 2011, 439: 479–485
Kimura Y, Goto YI, Kimura H. Antioxid Redox Sign, 2010, 12: 1–13
Kobayashi H, Ogawa M, Alford R, Choyke P, Urano Y. Chem Rev, 2010, 110: 2620–2640
Qian Y, Karpus J, Kabil O, Zhang SY, Zhu HL, Banerjee R, Zhao J, He C. Nat Commun, 2011, 2: 495
Sasakura K, Hanaoka K, Shibuya N, Mikami Y, Kimura Y, Komatsu T, Ueno T, Terai T, Kimura H, Nagano T. J Am Chem Soc, 2011, 133: 18003–18005
Liu CR, Pan J, Li S, Zhao Y, Wu LY, Berkman CE, Whorton AR, Xian M. Angew Chem Int Ed, 2011, 50: 10327–10329
Wan QQ, Song YC, Li Z, Gao XH, Ma HM. Chem Commun, 2013, 49: 502–504
Mao GJ, Wei TT, Wang XX, Huan SY, Lu DQ, Zhang J, Zhang XB, Tan WH, Shen GL, Yu RQ. Anal Chem, 2013, 85: 7875–7881
Yang S, Qi Y, Liu CH, Wang YJ, Zhao YR, Wang LL, Li JS, Tan WH, Yang RH. Anal Chem, 2014, 86: 7508–7515
Lin VS, Lippert AR, Chang CJ. Proc Natl Acad Sci USA, 2013, 110: 7131–7135
Chen YC, Zhu CC, Yang ZH, Chen JJ, He YF, Jiao Y, He WJ, Qiu L, Cen JJ, Guo ZJ. Angew Chem Int Ed, 2013, 52: 1688–1691
Hong YN, Lam JW, Tang BZ. Chem Soc Rev, 2011, 40: 5361–5388
Hu RR, Leung NL, Tang BZ. Chem Soc Rev, 2014, 43: 4494–4562
Xue XD, Zhao YY, Dai LR, Zhang X, Hao XH, Zhang CQ, Huo SD, Liu J, Liu C, Kumar A, Chen WQ, Zou GZ, Liang XJ. Adv Mater, 2014, 26: 712–717
Yu Y, Feng C, Hong YN, Liu JZ, Chen SJ, Ng KM, Luo KQ, Tang BZ. Adv Mater, 2011, 23: 3298–3302
Ding D, Li K, Liu B, Tang BZ. Accounts Chem Res, 2013, 46: 2441–2453
Liu YY, Wang Z, Zhang GX, Zhang W, Zhang DQ, Jiang XY. Analyst, 2012, 137: 4654–4657
Chen WW, Li QZ, Zheng WS, Hu F, Zhang GX, Wang Z, Zhang DQ, Jiang XY. Angew Chem Int Ed, 2014, 53: 13734–13739
Zhang L, Liu WW, Huang XH, Zhang GX, Wang XF, Wang Z, Zhang DQ, Jiang XY. Analyst, 2015, 140: 5849–5854
Huang XH, Gu XG, Zhang GX, Zhang DQ. Chem Commun, 2012, 48: 12195–12197
Moore PK, Bhatia M, Moochhala S. Trends Pharmacol Sci, 2003, 24: 609–611
Dombkowski RA, Russell MJ, Olson KR. Am J Physiol-Reg I, 2004, 286: R678–R685
Lin VS, Chang CJ. Curr Opin Chem Biol, 2012, 16: 595–601
Hong YN, Lam JWY, Tang BZ. Chem Commun, 2009, 29: 4332–4353
Lutolf MP, Tirelli N, Cerritelli S, Cavalli L, Hubbell JA. Bioconjugate Chem, 2001, 12: 1051–1056
Lv X, Liu J, Liu Y, Zhao Y, Sun YQ, Wang P, Guo W. Chem Commun, 2011, 47: 12843–12845
Leung CWT, Hong YN, Chen SJ, Zhao EG, Lam JWY, Tang BZ. J Am Chem Soc, 2012, 135: 62–65
Hu QL, Gao M, Feng GX, Liu B. Angew Chem Int Ed, 2014, 53: 14225–14229
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Huang, X., Liu, W. et al. Organic nanoparticles formed by aggregation-induced fluorescent molecules for detection of hydrogen sulfide in living cells. Sci. China Chem. 59, 106–113 (2016). https://doi.org/10.1007/s11426-015-5543-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-015-5543-2