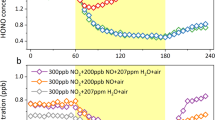
Abstract
Nitrous acid (HONO), as a primary precursor of OH radicals, has been considered one of the most important nitrogen-containing species in the atmosphere. Up to 30% of primary OH radical production is attributed to the photolysis of HONO. However, the major HONO formation mechanisms are still under discussion. During the Campaigns of Air Quality Research in Beijing and Surrounding Region (CAREBeijing2006) campaign, comprehensive measurements were carried out in the megacity Beijing, where the chemical budget of HONO was fully constrained. The average diurnal HONO concentration varied from 0.33 to 1.2 ppbv. The net OH production rate from HONO, P OH(HONO)net, was on average (from 05:00 to 19:00 h) 7.1 × 106 molecule/(cm3 s), 2.7 times higher than from O3 photolysis. This production rate demonstrates the important role of HONO in the atmospheric chemistry of megacity Beijing. An unknown HONO source (P unknown) with an average of 7.3 × 106 molecule/(cm3 s) was derived from the budget analysis during daytime. P unknown provided four times more HONO than the reaction of NO with OH did. The diurnal variation of P unknown showed an apparent photo-enhanced feature with a maximum around 12:00 h, which was consistent with previous studies at forest and rural sites. Laboratory studies proposed new mechanisms to recruit NO2 and J(NO2) in order to explain a photo-enhancement of of P unknown. In this study, these mechanisms were validated against the observation-constraint P unknown. The reaction of exited NO2 accounted for only 6% of P unknown, and P unknown poorly correlated with [NO2] (R = 0.26) and J(NO2)[NO2] (R = 0.35). These results challenged the role of NO2 as a major precursor of the missing HONO source.
Similar content being viewed by others
References
Kleffmann J, Gavriloaiei T, Hofzumahaus A, Holland F, Koppmann R, Rupp L, Schlosser E, Siese M, Wahner A. Daytime formation of nitrous acid: a major source of OH radicals in a forest. Geophys Res Lett, 2005, 32, doi: 10.1029/2005GL022524
Su H, Cheng YF, Shao M, Gao DF, Yu ZY, Zeng LM, Slanina J, Zhang YH, Wiensohler A. Nitrous acid (HONO) and its daytime sources at a rural site during the 2004 PRIDE-PRD experiment in China. J Geophys Res, 2008, 113, doi: 10.1029/2007JD009060.
Zhou XL, Beine HJ, Honrath RE, Fuentes JD, Simpson W, Shepson PB, Bottenheim JW. Snowpack photochemical production of HONO: a major source of OH in the Arctic boundary layer in springtime. Geophys Res Lett, 2001, 28: 4087–4090
Acker K, Möller D, Wieprecht W, Meixner FX, Bohn B, Gilge S, Plass-Dülmer C, Berresheim H. Strong daytime production of OH from HNO2 at a rural mountain site. Geophys Res Lett, 2006, 33, doi: 10.1029/2005GL024643
Alicke B, Platt U, Stutz J. Impact of nitrous acid photolysis on the total hydroxyl radical budget during the limitation of oxidant production/pianura padana produzione di ozono study in Milan. J Geophys Res, 2002, 107: LOP 9-1–LOP 9-17
Perner D, Platt U. Detection of nitrous acid in the atmosphere by differential optical absorption. Geophys Res Lett, 1979, 6: 917–920
Winer AM, Biermann HW. Long pathlength differential optical-absorption spectroscopy (DOAS) measurements of gaseous HONO, NO2 and HCHO in the California south coast air basin. Res Chem Intermediat, 1994, 20: 423–445
Ren XR, Harder H, Martinez M, Lesher RL, Oliger A, Simpas JB, Brune WH, Schwab JJ, Demerjian KL, He Y, Zhou XL, Gao HL. OH and HO2 chemistry in the urban atmosphere of New York City. Atmos Environ, 2003, 37: 3639–3651
Zhang, YH, Su H, Zhong LJ, Cheng YF, Zeng LM, Wang XS, Xiang YR, Wang JL, Gao DF, Shao M, Fan SJ, Liu SC. Regional ozone pollution and observation-based approach for analyzing ozone-precursor relationship during the PRIDE-PRD2004 campaign. Atmos Environ, 2008, 42: 6203–6218
Kleffmann J, Lörzer JC, Wiesen P, Kern C, Trick S, Volkamer R, Rodenas M, Wirtz K. Intercomparison of the DOAS and LOPAP techniques for the detection of nitrous acid (HONO). Atmos Environ, 2006, 40: 3640–3652
Stutz J, Alicke B, Neftel A. Nitrous acid formation in the urban atmosphere: gradient measurements of NO2 and HONO over grass in Milan, Italy. J Geophys Res, 2002, 107: LOP 5-1–LOP 5-15
Kessler C. Gasfoennige salpetrige saeure (HNO2) in der belasteten atmosphaere. Doctor Dissertation. Cologne: University of Cologne, 1984
Kirchstetter WT, Harley AR. Measurement of nitrous acid in motor vehicle exhaust. Environ Sci Technol, 1996, 30: 2843–2849
Ackermann R. Auswirkung von kraftfahrzeugemissionen in der urbanen atmosphäre. Doctor Dissertation. Heidelberg: Heidelberg University, 2000
Kurtenbach R, Becker KH, Gomes JAG, Kleffmann J, Lörze JC, Spittler M, Wiesen P, Ackermann R, Geyer A, Platt U. Investigations of emissions and heterogeneous formation of HONO in a road traffic tunnel. Atmos Environ, 2001, 35: 3385–3394
Alicke B, Geyer A, Hofzumahaus A, Holland F, Konrad S, Pätz HW, Schäfer J, Stutz J, Volz-Thomas A, Platt U. OH formation by HONO photolysis during the BERLIOZ experiment. J Geophys Res, 2003, 108: PHO 3-1–PHO 3-17
Stutz J, Alicke B, Ackermann R, Geyer A, Wang S, White AB, Williams EJ, Spicer CW, Fast JD. Relative humidity dependence of HONO chemistry in urban areas. J Geophys Res, 2004, 109, doi:10.1029/2003JD004135
Kleffmann J. Daytime sources of nitrous acid (HONO) in the atmospheric boundary layer. ChemPhysChem, 2007, 8: 1137–1144
Sarwar G, Roselle SJ, Mathur R, Appel W, Dennis RL, Vogel B. A comparison of CMAQ HONO predictions with observations from the northeast oxidant and particle study. Atmos Environ, 2008, 42: 5760–5770
Li X, Brauers T, Haeseler R, Bohn B, Fuchs H, Hofzumahaus A, Holland F, Lou S, Lu KD, Rohrer F, Hu M, Zeng LM, Zhang YH, Garland RM, Su H, Nowak A, Wiedensohler A, Takegawa N, Shao M, Wahner A. Exploring the atmospheric chemistry of nitrous acid (HONO) at a rural site in Southern China. Atmos Chem Phys, 2012, 12: 1497–1513
Akimoto H, Takagi H, Sakamaki F. Photoenhancement of the nitrous acid formation in the surface reaction of nitrogen dioxide and water vapour: extra radical source in smog chamber experiments. Int J Chem Kinet, 1987, 19: 539–551
Rohrer F, Bohn B, Brauers T, Brüning D, Johnen FJ, Wahner A, Kleffmann J. Characterisation of the photolytic HONO-source in the atmosphere simulation chamber SAPHIR. Atmos Chem Phys, 2005, 5: 2189–2201
Stemmler K, Ammann M, Donders C, Kleffmann J, George C. Photosensitized reduction of nitrogen dioxide on humic acid as a source of nitrous acid. Nature, 2006, 440: 195–198
Zádor J, Turányi T, Wirtz K, Pilling MJ. Measurement and investigation of chamber radical sources in the European Photoreactor (EUPHORE). J Atmos Chem, 2006, 55: 147–166
Li SP, Matthews J, Sinha A. Atmospheric hydroxyl radical production from electronically exited NO2 and H2O. Science, 2008, 319: 1657–1660
Langridge JM, Gustafsson RJ, Griffiths PT, Cox RA, Lambert RM, Jones RL. Solar driven nitrous acid formation on building material surfaces containing titanium dioxide: a concern for air quality in urban areas. Atmos Environ, 2009, 43: 5128–5131
Monge ME, D’Anna B, Mazri L, Giroir-Fendler A, Ammann M, Donaldson DJ, George C. Light changes the atmospheric reactivity of soot. Proc Natl Acad Sci USA, 2010, 107: 6605–6609
Finlayson-Pitts BJ, Wingen LM, Sumner AL, Syomin D, Ramazan KA. The heterogeneous hydrolysis of NO2 in laboratory systems and in outdoor and indoor atmospheres: an integrated mechanism. Phys Chem Chem Phys, 2003, 5: 223–242
Su H, Cheng Y, Oswald R, Behrendt T, Trebs I, Meixner FX, Andreae MO, Cheng P, Zhang Y, Poeschl U. Soil nitrite as a source of atmospheric HONO and OH radicals. Science, 2011, 333: 1616–1618
Su H, Cheng Y, Pöschl U. The exchange of soil nitrite and atmospheric HONO: a missing process in the nitrogen cycle and atmospheric chemistry. In: Barnes I, Rudziński KJ. Disposal of Dangerous Chemicals in Urban Areas and Mega Cities. Berlin: Springer, 2013. 93–99
Costabile F, Amoroso A, Wang F. Sub-μm particle size distributions in a suburban mediterranean area. Aerosol populations and their possible relationship with HONO mixing ratios. Atmos Environ, 2010, 44: 5258–5268
Su H. HONO: a study to its sources and impacts from field measurements at the sub-urban areas of PRD region. Doctor Dissertation. Beijing: Peking University, 2008
Hofzumahaus A, Rohrer F, Lu KD, Boh B, Brauers T, Chang CC, Fuchs H, Holland F, Kita K, Kondo Y, Li X, Lou SR, Shao M, Zeng LM, Wahner A, Zhang YH. Amplified trace gas removal in the troposphere. Science, 2009, 324: 1702–1704
Zhou XL, He Y, Huang G, Thornberry TD, Carroll MA, Bertman SB. Photochemical production of nitrous acid on glass sample manifold surface. Geophys Res Lett, 2002, 29: 26-1–26-4
Heland J, Kleffmann J, Kurtenbach R, Wiesen P. A new instrument to measure gaseous nitrous acid (HONO) in the atmosphere. Environ Sci Technol, 2001, 35: 3207–3212
Schlosser E, Brauers T, Dorn HP, Fuchs H, Haseler R, Hofzumahaus A, Holland F, Wahner A, Kanaya Y, Kajii Y, Miyamoto K, Nishida S, Watanabe K, Yoshino A, Kubistin D, Martinez M, Rudolf M, Harder H, Berresheim H, Elste T, Plass-Dulmer C, Stange G, Schurath U. Techincal note: formal blind intercomparison of OH measurements: results from the international campaign HOxComp. Atmos Chem Phys, 2009, 9: 7923–7948
Lu KD, Hofzumahaus A, Holland F, Bohn B, Brauers T, Fuchs H, Hu M, Haseler R, Kita K, Kondo Y, Li X, Lou SR, Oebel A, Shao M, Zeng LM, Wahner A, Zhu T, Zhang YH, Rohrer F. Missing OH source in a suburban environment near Beijing: observed and modeled OH and HO2 concentrations in summer 2006. Atmos Chem Phys, 2013, 13: 1057–1080
Bohn B, Corlett GK, Gillman M, Sanghavi S, Stange G, Tensing E, Vrekoussis M, Bloss WJ, Clapp LJ, Kortner M, Corn HP, Monks PS, Platt U, Plass-Dulmer C, Mihalopoulos N, Heard DE, Clemitshaw KC, Meixner FX, Prevot ASH, Schmitt R. Photolysis frequency measurement techniques: results of comparison within the ACCENT project. Atmos Chem Phys, 2008, 8: 5373–5391
Takegawa N, Miyakawa T, Kondo Y, Jimenez JL, Zhang Q, Worsnop DR, Fukuda M. Seasonal and diurnal variations of submicron organic aerosol in Tokyo observed using the Aerodyne aerosol mass spectrometer. J Geophys Res, 2006, 111, doi: 10.1029/2005JD006515
Wiedensohler A, Cheng YF, Nowak A, Wehner B, Achtert P, Berghof M, Birmili W, Wu ZJ, Hu M, Zhu T, Takegawa N, Kita K, Kondo Y, Lou SR, Hofzumahaus A, Holland F, Wahner A, Gunthe SS, Rose D, Su H, Poeschl U. Rapid aerosol particle growth and increase of cloud condensation nucleus activity by secondary aerosol formation and condensation: a case study for regional air pollution in northeastern China. J Geophys Res, 2009, 114, doi: 10.1029/2008JD010884
Cheng YF, Berghof M, Garland RM, Wiedensohler A, Wehner B, Mueller T, Su H, Zhang YH, Achtert P, Nowak A, Poeschl U, Zhu T, Hu M, Zeng LM. Influence of soot mixing state on aerosol light absorption and single scattering albedo during air mass aging at a polluted regional site in northeastern China. J Geophys Res, 2009, 114, doi: 10.1029/2008JD010883
Slanina J, Wyers GP. Monitoring of atmospheric components by automatic denuder systems. J Anal Chem, 1994, 350: 467–473
Oms MT, Jongejan PAC, Veltkamp AC, Wyers GP, Slanina J. Continuous monitoring of atmospheric HCl, HNO2, HNO3 and SO2 by wet-annular denuder air sampling with on-line chromatographic analysis. Int J Environ An Ch, 1996, 62: 207–218
Hu M, Zhou FM, Shao KS, Zhang YH, Tang XY, Slanina J. Diurnal variations of aerosol chemical compostions and related gaseous pollutants in Beijing and Guangzhou. J Environ Sci Heal A, 2002, 37: 479–488
Zhang G, Slanina S, Boring CB, Jongejan PAC, Dasgupta PK. Continuous wet denuder measurements of atmospheric nitric and nitrous acids during the 1999 Atlanta Supersite. Atmos Environ, 2003, 37: 1351–1364
Trebs I, Meixner FX, Slanina J, Otjes R, Jongejan P, Andreae MO. Real-time measurements of ammonia, acidic trace gases and water-soluble inorganic aerosol species at a rural site in the Amazon Basin. Atmos Chem Phys, 2004, 4: 967–987
Trebs I, Lara LL, Zeri LMM, Gatti LV, Artaxo P, Dlugi R, Slanina J, Andreae MO, Meixner FX. Dry and wet deposition of inorganic nitrogen compounds to a tropical pasture site (Rondonia, Brazil). Atmos Chem Phys, 2006, 6: 447–469
Kleffmann J, Wiesen P. Technical note: quantification of interferences of wet chemical HONO LOPAP measurements under simulated polar conditions. Atmos Chem Phys, 2008, 8: 6813–6822
Wang HP, Zhou B, Chen LM. Monitoring HONO of the atmosphere by differential optical absorption spectroscopy. J Fudan University (Natural Science), 2004, 43: 604–609
Acker K, Febo A, Trick S, Perrino C, Bruno P, Wiesen P, Möller D, Wieprecht W, Auel R, Guisto M, Geyer A, Platt U, Allegrini I. Nitrous acid in the urban area of Rome. Atmos Environ, 2006, 40: 3123–3133
Acker K, Möller D. Atmospheric variation of nitrous acid at different sites in Europe. Environ Chem, 2007, 4: 242–255
Yu Y, Galle B, Panday A, Hodson E, Prinn R, Wang S. Observations of high rates of NO2-HONO conversion in the nocturnal atmospheric boundary layer in Kathmandu, Nepal. Atmos Chem Phys, 2009, 9: 6401–6415
Kleffmann J, Kurtenbach R, Lörzer J, Wiesen P, Kalthoff N, Vogel B, Vogel H. Measured and simulated vertical profiles of nitrous acid. Part I: field measurements. Atmos Environ, 2003, 37: 2949–2955
Zhou FM, Shao KS, Hu M, Tang XY. The hourly measurement of aerosol and related gases in Guangzhou. Acta Scientiarum Naturalium Universitatis Pekinensis, 2002, 38: 185–191
Qin M, Xie PH, Liu WQ, Li A, Dou K, Fang W, Liu HG, Zhang WJ. Observation of atmospheric nitrous acid with DOAS in Beijing, China. J Environ Sci-China, 2006, 18: 69–75
Qin M, Xie PH, Su H, Gu JW, Peng FM, Li SW, Zeng LM, Liu JG, Liu WQ, Zhang YH. An observational study of the HONO-NO2 coupling at an urban site in Guangzhou City, South China. Atmos Environ, 2009, 43: 5731–5742
Zhou XL, Huang G, Civerolo K, Roychowdhury U, Demerjian KL. Summertime observations of HONO, HCHO, and O3 at the summit of Whiteface Mountain, New York. J Geophys Res, 2007, 112, doi: 10.1029/2006JD007256
Su H, Cheng YF, Cheng P, Dong SF, Zeng LM, Wang XS, Slanina J, Shao M, Wiensohler A. Observation of nighttime nitrous acid (HONO) formation at a non-urban site during PRIDE-PRD2004 in China. Atmos Environ, 2008, 42: 6219–6232
Febo A, Perrino C, Allegrini I. Measurement of nitrous acid in Milan, Italy, by DOAS and diffusion denuders. Atmos Environ, 1996, 30: 3599–3609
Zhou XL, Civerolo K, Dai HP, Huang G, Schwab J, Demerjian K. Summertime nitrous acid chemistry in the atmospheric boundary layer at a rural site in New York State. J Geophys Res, 2002, 107, 4590, doi: 10.1029/2001JD001539
Ramazan KA, Syomin D, Finlayson-Pitts BJ. The photochemical production of HONO during the heterogeneous hydrolysis of NO2. PhysChemChemPhys, 2004, 6: 3836–3843
Reisinger AR. Observations of HNO2 in the polluted winter atmosphere: possible heterogeneous production on aerosols. Atmos Environ, 2000, 34: 3856–3874
Vogel B, Vogel H, Kleffmann J, Kurtenbach R. Measured and simulated vertical profiles of nitrous acid. Part II. Model simulations and indications for a photolytic source. Atmos Environ, 2003, 37: 2957–2966
Carr S, Heard DE, Blitz MA. Comment on “atmospheric hydroxyl radical production from electronically excited NO2 and H2O”. Science, 2009, 324: 5925
Stuhl F, Niki H. Flash photochemical study of the reaction OH + NO + M using resonance fluorescent detection of OH. J Chem Phys, 1972, 57: 3677–3679
Stockwell WR, Kirchner F, Kuhn M, Seefeld S. A new mechanism for regional atmospheric chemistry modeling. J Geophys Res, 1997, 102: 25847–25879
Atkinson R, Baulch DL, Cox RA, Crowley JN, Hampson Jr RF, Kerr JA, Rossi MJ, Troe J. Summary of evaluated kinetic and photochemical data for atmospheric chemistry. Not in System, 2001: 1–56
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Q., Su, H., Li, X. et al. Daytime HONO formation in the suburban area of the megacity Beijing, China. Sci. China Chem. 57, 1032–1042 (2014). https://doi.org/10.1007/s11426-013-5044-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-013-5044-0