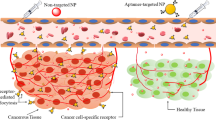
Abstract
Early detection and treatment of cancer depends on developing highly sensitive and specific methods for targeting cancer cells. To do this, aptamers, which are generated by a novel technique called cell-SELEX (systematic evolution of ligands by exponential enrichment), have been widely applied in cancer cell targeting based on such merits as high target affinity and specificity, small size, minimal immunogenicity, and ease of chemical modification. Furthermore, aptamers can gain more flexibility as cancer cell targeting tools when conjugated to nanomaterials, including metallic nanoparticles, quantum dots, silica nanoparticles, and carbon nanomaterials, among others. In this review, we discuss the use of cell-SELEX-based aptamer-nanomaterials conjugates as novel molecular tools for enhanced targeting of cancer cells.
Similar content being viewed by others
References
Jemal, A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. CA Cancer J Clin, 2005, 55(1): 10–30
Liu J, Cao Z, Lu Y. Functional nucleic acid sensors. Chem Rev, 2009, 109(5): 1948–1998
Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science, 1990, 249(4968): 505–510
Ellington AD, Szostak JW. In vitro selection of RNA that bind specific ligands. Nature, 1990, 346: 818–822
Osborne SE, Matsumura I, Ellington AD. Aptamers as therapeutic and diagnostic reagents: problems and prospects. Curr Opin Chem Biol, 1997, 1(1): 5–9
Famulok M, Hartig JS, Mayer G. Functional aptamers and aptazymes in biotechnology, diagnostics, and therapy. Chem Rev, 2007, 107(9): 3715–3743
Daniels DA, Chen H, Hicke BJ, Swiderek KM, Gold L. A tenascin-C aptamer identified by tumor CELL SELEX: systematic evolution of ligands by exponential enrichment. Proc Natl Acad Sci USA, 2003, 100(26): 15416–15421
Shamah SM, Healy JM, Cload ST. Complex target SELEX. Acc Chem Res, 2008, 41(1): 130–138.
Shangguan D, Li Y, Tang Z, Cao Z, Chen H, Mallikaratchy P, Sefah K, Yang C, Tan W. Aptamers evolved from live cells as effective molecular probes for cancer study. Proc Natl Acad Sci USA, 2006, 103(32): 11838–11843
Berezovski MV, Lechmann M, Musheev MU, Mak TW, Krylov SN. Aptamer-facilitated biomarker discovery (AptaBiD). J Am Chem Soc, 2008, 130(28): 9137–9143
Fang X, Tan W. Aptamers generated from Cell-SELEX for molecular medicine: A chemical biology approach. Acc Che Res, 2010, 43(1): 48–57
Wang Z, Lu Y. Functional DNA directed assembly of nanomaterials for biosensing. J Mater Chem, 2009, 19: 1788–1798
Chen T, Shukoor MI, Chen Y, Yuan Q, Zhu Z, Zhao Z, Gulbakan B, Tan W. Aptamer-conjugated nanomaterials for bioanalysis and biotechnology applications. Nanoscale, 2011, 3: 546–556
Wang J. Nanomaterial-based amplified transduction of biomolecular interactions. Small, 2005, 1(11): 1036–1043
Kim Y, Cao Z, Tan W. Molecular assembly for high-performance bivalent nucleic acid inhibitor. Proc Natl Acad Sci USA, 2008, 105(15): 5664–5669
Wu Y, Phillips JA, Liu H, Yang R, Tan W. Carbon nanotubes protect DNA strands during cellular delivery. ACS Nano, 2008, 2(10): 2023–2028
Ireson CR, Kelland LR. Discovery and development of anticancer aptamers. Mol Cancer Ther, 2006, 5: 2957–2962
Lupold SE, Hicke BJ, Lin Y, Coffey DS. Identification and characterization of nuclease-stabilized RNA molecules that bind human prostate cancer cells via the prostate-specific membrane antigen. Cancer Res, 2002, 62: 4029–4033
Pu Y, Zhu Z, Liu H, Zhang J, Liu J, Tan W. Using aptamers to visualize and capture cancer cells. Anal Bioanal Chem, 2010, 397(8): 3225–3233
Tang Z, Shangguan D, Wang K, Shi H, Sefah K, Mallikratchy P, Chen HW, Li Y, Tan W. selection of aptamers for molecular recognition and characterization of cancer cells. Anal Chem, 2007, 79(13): 4900–4907
Chen HW, Medley CD, Sefah K, Shangguan D, Tang Z, Meng L, Smith JE, Tan W. Molecular recognition of small-cell lung cancer cells using aptamers. ChemMedChem, 2008, 3: 991–1001
Sefah K, Tang Z, Shangguan D, Chen H W, Lopez-Colon D, Li Y, Parekh P, Martin J, Meng L, Phillips JA, Kim YM, Tan W. Molecular recognition of acute myeloid leukemia using aptamersMolecular recognition of AML. Leukemia, 2009, 23: 235–244
Ghosh SK, Pal T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: from theory to applications. Chem Rev, 2007, 107(11): 4797–4862
Medley CD, Smith JE, Tang ZW, Wu YR, Bamrungsap S, Tan WH. Gold nanoparticle-based colorimetric assay for the direct detection of cancerous cells. Anal Chem, 2008, 80(11): 1067–1072
Huang YF, Lin YW, Lin ZH, Chang HT. Aptamer-modified gold nanoparticles for targeting breast cancer cells through light scattering. J Nanopart Res, 2009, 11: 775–783
Liu G, Mao X, Phillips JA, Xu H, Tan W, Zeng L. Aptamer-nanoparticle strip biosensor for sensitive detection of cancer cells. Anal Chem, 2009, 81(24): 10013–10018
Lu W, ArumugamS R, Senapati D, Singh AK, Arbneshi T, Khan SA, Yu H, Ray PC. Multifunctional oval-shaped gold-nanoparticle-based selective detection of breast cancer cells using simple colorimetric and highly sensitive two-photon scattering assay. ACS Nano, 2010, 4(3): 1739–1749
Jain PK, Huang X, El-Sayed IH, El-Sayed MA. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc Chem Res, 2008, 41(12): 1578–1586
Cheon J, Lee JH. Synergistically integrated nanoparticles as multimodal probes for nanobiotechnology. Acc Chem Res, 2008, 41(12): 1630–1640
Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnol, 2007, 2: 751–760
Sarkar B, Dosch J, Simeone DM. Cancer stem cells: A new theory regarding a timeless disease. Chem Rev, 2009, 109(7): 3200–3208
Lorenzo LR, Javier F, Abajo G, Liz-Marzn LM. Surface enhanced raman scattering using star-shaped gold colloidal nanoparticles. J Phys Chem C, 2010, 114(16): 7336–7340
Khoury CG, Vo-Dinh T. Gold nanostars for surface-enhanced raman scattering: synthesis, characterization and optimization. J Phys Chem C, 2008, 112(48): 18849–18859
Lu W, Singh AK, Khan SA, Senapati D, Yu H, Ray PC. Gold nano-popcorn-based targeted diagnosis, nanotherapy treatment, and in situ monitoring of photothermal therapy response of prostate cancer cells using surface-enhanced raman spectroscopy. J Am Chem Soc, 2010, 132(51): 18103–18114
Huang YF, Chang HT, Tan WH. Cancer cell targeting using multiple aptamers conjugated on nanorods. Anal Chem, 2008, 80(3): 567–572
Jain PK, Huang X, El-Sayed IH, El-Sayed MA. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc Chem Res, 2008, 41(12): 1578–1586
Huang YF, Sefah K, Bamrungsap S, Chang HT, Tan WH. Selective photothermal therapy for mixed cancer cells using aptamer-conjugated nanorods. Langmuir, 2008, 24(20): 11860–11865
Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science, 2005, 307(5709): 538–544
Zhang J, Jia X, Lv XJ, Deng YL, Xie HY. Fluorescent quantum dot-labeled aptamer bioprobes specifically targeting mouse liver cancer cells. Talanta, 2010, 81(1–2): 505–509
Li Z, Huang P, He R, Lin J, Yang S, Zhang X, Ren Q, Cui D. Aptamer-conjugated dendrimer-modified quantum dots for cancer cell targeting and imaging. Mater Lett, 2010, 64(3): 375–378
Bagalkot V, Zhang L, Levy-Nissenbaum E, Jon S, Kantoff PW, Langer R, Farokhzad OC. Quantum dot-aptamer conjugates for synchronous cancer imaging, therapy, and sensing of drug delivery based on bi-fluorescence resonance energy transfer. Nano Lett, 2007, 7(10): 3065–3070
Savla R, Taratula O, Garbuzenko O, Minko T. Tumor targeted quantum dot-mucin 1 aptamer-doxorubicin conjugate for imaging and treatment of cancer. J Control Release, 2011, doi:10.1016/j.jconrel. 2011.02.01
Ko MH, Kim S, Kang WJ, Lee JH, Kang H, Moon SH, Hwang DW, Ko HY, Lee DS. In vitro derby imaging of cancer biomarkers using quantum dots. Small, 2009, 5(10): 1207–1212
Kang WJ, Chae JR, Cho YL, Lee JD, Kim S. Multiplex imaging of single tumor cells using quantum-dot-conjugated aptamers. Small, 2009, 5(22): 2519–2522
Jie G, Wang L, Yuan J, Zhang S. Versatile electrochemiluminescence assays for cancer cells based on dendrimer/Cdse-Zns-quantum dot nanoclusters. Anal Chem, 2011, 83(10): 3873–3880
Joshua E. Smith, Lin Wang, Tan W. Bioconjugated silica-coated nanoparticles for bioseparation and bioanalysis. Trends Anal Chem, 2006, 25(9): 848–855
Yan J, Estévez MC, Smith JE, Wang K, He X, Wang L, Tan W. Dye-doped nanoparticles for bioanalysis. Nano Today, 2007, 2(3): 44–50
Zhao XJ, Hilliard LR, Mechery SJ, Wang YP, Bagwe RP, Jin SG, Tan WH. A rapid bioassay for single bacterial cell quantitation using bioconjugated nanoparticles. Proc Natl Acad Sci USA, 2004, 101(42): 15027–15032
Deng T, Li J, Zhang LL, Jiang JH, Chen JN, Shen GL, Yu RQ. A sensitive fluorescence anisotropy method for the direct detection of cancer cells in whole blood based on aptamer-conjugated near-infrared fluorescent nanoparticles. Biosens Bioelectron, 2010, 25(7): 1587–1591
Herr JK, Smith JE, Medley CD, Shangguan D, Tan W. Aptamer-conjugated nanoparticles for selective collection and detection of cancer cells. Anal Chem, 2006, 78(9): 2918–2924
Smith JE, Medley CD, Tang Z, Shangguan D, Lofton C, Tan W. Aptamer-conjugated nanoparticles for the collection and detection of multiple cancer cells. Anal Chem, 2007, 79(8): 3075–3082
Medley CD, Bamrungsap S, Tan W, Smith JE. Aptamer-conjugated nanoparticles for cancer cell detection. Anal Chem, 2011, 83(3): 727–734
Estévez MC, O’Donoghue MB, Chen X, Tan W. Highly fluorescent dye-doped silica nanoparticles increase flow cytometry sensitivity for cancer cell monitoring. Nano Res, 2009, 2(6): 448–461
Chen X, Estévez MC, Zhu Z, Huang YF, Chen Y, Wang L, Tan W. Using aptamer-conjugated fluorescence resonance energy transfer nanoparticles for multiplexed cancer cell monitoring. Anal Chem, 2009, 81(16): 7009–7014
Jun YW, Seo JW, Cheon J. Nanoscaling laws of magnetic nanoparticles and their applicabilities in biomedical sciences. Acc Chem Res, 2008, 41(2): 179–189
Wang L, Zhao W, Tan W. Bioconjugated silica nanoparticles: Development and applications. Nano Res, 2008, 1(2): 99–115
Hwang DW, Ko HY, Lee JH, Kang H, Ryu SH, Song IC, Lee DS, Kim S. A nucleolin-targeted multimodal nanoparticle imaging probe for tracking cancer cells using an aptamer. J Nucl Med, 2010, 51(1): 98–105
Zheng M, Jagota A, Semke ED, Diner BA, McLean RS, Lustig SR, Richardson RE, Tassi NG. DNA-assisted dispersion and separation of carbon nanotubes. Nat Mater, 2003, 2: 338–342
Zheng M, Jagota A, Strano MS, Santos AP, Barone P, Chou SG, Diner BA, Dresselhaus MS, McLean RS, Onoa GB, Samsonidze GG, Semke ED, Usrey M, Walls DJ. Structure-based carbon nanotube sorting by sequence-dependent dna assembly. Science, 2003, 302(5650): 1545–1548
Nguyen CV, Delzeit L, Cassell AM, Li J, Han J, Meyyappan M. Preparation of nucleic acid functionalized carbon nanotube arrays. Nano Lett, 2002, 2(10): 1079–1081
Hazani M, Naaman R, Hennrich F, Kappes MM. Confocal fluorescence imaging of DNA-functionalized carbon nanotubes. Nano Lett, 2003, 3(2): 153–155
Taghdisi SM, Lavaee P, Ramezani M, Abnous K. Reversible Targeting and controlled release delivery of daunorubicin to cancer cells by aptamer-wrapped carbon nanotubes. Eur J Pharm Biopharm, 2011, 77(2): 200–206
Feng L, Chen Y, Ren J, Qu X. A graphene functionalized electrochemical aptasensor for selective label-free detection of cancer cells. Biomaterials, 2011, 32(11): 2930–2937
Cao Z, Tong R, Mishra A, Xu W, Wong GCL, Cheng J, Lu Y. Reversible cell-specific drug delivery with aptamer-functionalized liposomes. Angew Chem Int Ed, 2009, 48(35): 6494–6498
Kang H, O’Donoghue MB, Liu H, Tan W. A liposome-based nanostructure for aptamer directed delivery. Chem Commun, 2010, 46(2): 249–251
Wu Y, Sefah K, Liu H, Wang R, Tan W. DNA aptamer-micelle as an efficient detection/delivery vehicle toward cancer cells. Proc Natl Acad Sci USA, 2010, 107(1): 5–10
Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW, Richie JP, Langer R. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci USA, 2006, 103(16): 6315–6320
Dhar S, Gu FX, Langer R, Farokhzad OC, Lippard SJ. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticles. Proc Natl Acad Sci USA, 2008, 105(45): 17356–17361
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kong, R., Chen, Z., Ye, M. et al. Cell-SELEX-based aptamer-conjugated nanomaterials for enhanced targeting of cancer cells. Sci. China Chem. 54, 1218–1226 (2011). https://doi.org/10.1007/s11426-011-4336-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-011-4336-5