Abstract
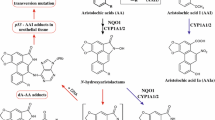
Aristolochic acid (AA), a mixture of structure-related nitrophenanthrene carboxylic acid derivatives derived from Aristolochia spp, is associated with nephrotoxin and carcinogen. AA-DNA adducts induced by reductive metabolic activation of AA were detected in tissues of animals and in patients exposed to AA. The DNA adducts were generally used as biomarkers in toxicological study of AA. In this short review, quantitative analysis of AA-DNA adducts in various in vitro and in vivo systems by using 32P-postlabelling assay, HPLC-UV, HPLC-radiation monitor, HPLC-FLD, HPLC-ESI/MS and UPLC-MS/MS methods is discussed. The distribution of AA-DNA adducts in various tissues is also summarized.
Similar content being viewed by others
References
Rucker G, Chung B S. Aristolochic acid from Aristolochia manshuriensi. Planta Med, 1975, 27: 68–71
Priestap H A. Minor aristolochic acids from Aristolochia argetina and mass spectral analysis of aristolochic acids. Phytochemistry, 1987, 26: 519–529
Kupchan S M, Doskotch R W. Tumor inhibitors. I. Aristolochic acid, the active principle of Aristolochia indica. J Med Chem, 1962, 5: 657–659
Kumar V, Ponam D, Prasad A K, Parmar V S. Naturally occurring aristolactams, aristolochic acids and diaxoapophines and their biological activities. Nat Prod Rep, 2003, 20: 565–583
Kluthe R, Vogt A, Batsford S. Doppelblindstudie zur beeinflussung der phagocytosefahigkeit von granulocyten durch Aristolochiasaure. Drug Res, 1982, 32: 443–445
Mengs U, Lang W, Poch J A. The carcinogenic action of aristolochic acid in rats. Arch Toxicol, 1982, 107: 107–119
Mengs U. On the histopathogenesis of rat forestomach carcinoma caused by aristolochic acid. Arch Toxicol, 1983, 52: 209–220
Pfau W, Schmeiser H H, Wiessler M. N6-adenyl arylation of DNA by aristolochic acid II and a synthetic model for the putative proximate carcinogen. Chem Res Toxicol, 1991, 4: 581–586
Bieler C A, Stiborova M, Wiessler M, Cosyns J P, van Ypersele de Strihou C, Schmeiser H H. 32P-post-labelling analysis of DNA adducts formed by aristolochic acid in tissues from patients with Chinese herbs nephropathy. Carcingenesis, 1997, 18: 1063–1067
Stiborova M, Frei E, Sopko B, Wiessler M, Schmeiser H H. Carcinogenic aristolochic acids upon activation by DT-diaphorase form adducts found in DNA of patients with Chinese herbs nephropathy. Carcingenesis, 2002, 23: 617–625
Lord G M, Cook T, Arlt V M, Schmeiser H H, Williams G, Pusey C D. Urothelial malignant disease and Chinese herbal nephropathy. Lancet, 2001, 358: 1515–1516
Debelle F D, Nortier J L, de Prez E G, Garbar C H, Vienne A R, Salmon I J, Deschodt-Lanckman M M, Vanherweghem J L. Aristolochic acid induce chronic renal failure with interstitial fibrosis in salt-depleted rats. J Am Soc Nephrol, 2002, 13: 431–436
Cosyns J P, Dehoux J P, Guiot Y, Goebbels R M, Robert A, Bernard A M, van Ypersele de Strihou C. Chronic aristolochic acid toxicity in rabbits: A model of Chinese herbs nephropathy? Kidney Int, 2001, 59: 2164–2173
Mengs U. Tumor induction in mice following exposure to aristolochic acid. Arch Toxicol, 1988, 61: 504–505
Pfau W, Schmeiser H H, Wiessler M. Aristolochic acid binds covalently to the amino group of purine nucleotides in DNA. Carcinogenesis, 1990, 11: 313–319
Cosyns J P, Jadoul M, Squifflet J P, Wese F X, van Ypersele de Strihou C. Urothelial lesions in Chinese-herb nephropathy. Am J Kidney Dis, 1999, 33: 1011–1017
Nortier J L, Muniz M C, Schmeiser H H, Arlt V M, Bieler C A, Petein M, Depierreux M F, de Pauw L, Abramowicz D, Vereerstraeten P, Vanherweghem J L. Urothelial carcinoma associated with the use of Chinese herbs (Aristolochia species). N Engl J Med, 2000, 342: 1686–1692
Mengs U. Acute toxicity of aristolochic acid in rodents. Arch Toxicol, 1987, 59: 328–331
Lebeau C, Arlt V M, Schmeiser H H, Boom A, Verroust P J, Devuyst O, Beauwens R. Aristolochic acid impedes endocytosis and induces DNA adducts in proximal tubule cells. Kidney Int, 2001, 60: 1332–1342
Arlt V M, Alunni-Perret V, Quatrehomme G, Ohayon P, Albano L, Gaid H, Michiels J F, Meyrier A, Cassuto E, Wiessler M, Schmeiser H H, Cosyns J P. Aristolochic acid-DNA adduct as marker of AA exposure and risk factor for AA nephropathy-associated cancer. Int J Cancer, 2004, 111: 977–980
Cheng C L, Chen K J, Shih P H, Lu L Y, Hung C F, Lin W C, Gu J Y. Chronic renal failure rats are highly sensitive to aristolochic acids, which are nephrptpxic and carcinogenic agents. Cancer Lett, 2006, 232: 236–242
Chen L, Mei N, Yao L, Chen T. Mutations induced by carcinogenic doses of aristolochic acid in kidney of big blue transgenic rats. Toxicol Lett, 2006, 165: 250–256
Wu K Y, Jiang L P, Cao J, Yang G, Geng C Y, Zhong L F. Genotoxic effect and nitrative DNA damage in HepG2 cells exposed to aristolochic acid. Mutat Res, 2007, 630: 97–102
Chang H R, Lian J D, Lo C W, Huang H P, Wang C J. Aristolochic acid-induced cell cycle G1 arrest in human urothelium SV-HUC-1 cells. Food Chem Toxicol, 2007, 45: 396–402
Vanherweghem J L, Depierreux M, Tielemans C, Abramowicz D, Vanhaelen-Fastre R, Vanhaelen M. Rapidly progressive interstitial renal fibrosis in young women: Association with slimming regimen including Chinese herb. Lancet, 1993, 341: 381–391
Vanhaelen M, Vanhaelen-Fastre R, But P, Vanherweghem J L. Identication of aristolochic acid in Chinese herbs. Lancet, 1994, 343: 174
Martinez M C, Nortier J, Vereerstaeten P, Vanherweghem J L. Progression rate of Chinese herb nephropathy: Impact of Aristolochia Fangchi ingested dose. Nephrol Dial Transplant, 2002, 17: 408–412
Cosyns J P. Aristolochic acid and “Chinese herbs nephropathy“: A review of the evidence to date. Drug Saf, 2003, 26: 33–48
Schmeiser H H, Schoepe K B, Wiessler M. DNA adduct formation of aristolochic acid I and II in vitro and in vivo. Carcinogenesis, 1988, 9: 297–303
Pfau W, Schmeiser H H, Wiessler M. 32P-postlabelling analysis of the DNA adducts formed by aristolochic acid I and II. Carcinogenesis, 1990, 11: 1627–1633
Stiborova M, Fernando R C, Schmeiser H H, Frei E, Pfau W, Wiessler M. Characterization of DNA adducts formed by aristolochic acids in the target organ (forestomach) of rats by 32P-labelling analysis using different chromatographic procedures. Carcinogenesis, 1994, 15: 1187–1192
Stiborova M, Frei E, Breuer A, Wiessler M, Schmeiser H H. Evidence for reductive activation of carcinogeneic aristolochic acids by prostalandin H synthase-32P-postlabling analysis of DNA adduct formation. Mutat Res, 2001, 493: 149–160
Stiborova M, Frei E, Sopko B, Spokova K, Markova V, Lankova M, Kumstyrova T, Wiessler M, Schmeiser H H. Human cytosolic enzymes involved in the metabolic activation of carcinogeneic aristolochic acid: Evidence for reductive activation by human NADH: quinine oxidoreductase. Carcinogenesis, 2003, 24: 1695–1703
Schmeiser H H, Frei E, Wiessler M, Stiborova M. Comparison of DNA adduct formation by aristolochic acids in various in vitro activation systems by P-32-post-labelling: Evidence for reductive activation by peroxidases. Carcinogenesis, 1997, 18: 1055–1062
Arlt V M, Pfohl-Leszkowicz A, Cosyns J P, Schmeiser H H. Analysis of DNA adducts formed by ochratoxin A and aristolochic acid in patients with Chinese herbs nephropathy. Mutat Res, 2001, 494: 143–150
Chan W, Zheng Y F, Cai Z W. Liquid chromatography-tandem mass spectrometry analysis of the DNA adducts of aristolochic acids. J Am Soc Mass Spectrom, 2007, 18: 642–650
Stiborova M, Frei E, Arlt V M, Schmeiser H H. Metabolic activation of carcinogenic aristolochic acid, a risk factor for Balkan endemic nephropathy. Mutat Res, 2008, 658: 55–67
Randerath K, Randerach E, Agrawal H P, Gupta R C, Schurdak M E, Reddy M V. Postlabeling methods for carcinogen-DNA adduct analysis. Environ Health Perspect, 1985, 63: 57–65
Fernando R C, Schmeiser H H, Nicklas W, Wiessler M. Detection and quantitation of dG-AAI and dA-AAI adducts by 32P-postlabeling methods in urothelium and exfoliated cells in urine of rats treated with aristolochic acid I. Carcinogenesis, 1992, 13: 1835–1839
Mei N, Arlt V M, Phillips D H, Heflich R H, Chen T. DNA adduct formation and mutation induction by aristolochic acid in rat kidney and liver. Mutat Res, 2006, 602: 83–91
Dong H, Suzuki N, Torres M C, Bonala R R, Johnson F, Grollman A P, Shibutani S. Quantitative determination of aristolochic acid-derived DNA adducts in rats using 32P-postlabeling/polyacrylamide gel electrophoresis analysis. Am Soc Pharmacol Experiment Therapeut, 2006, 34: 1122–1127
Stiborova M, Frei E, Hodek P, Wiessler M, Schmeiser H H. Human hepatic and renal microsomes, cytochromes P450 1A1/2, NADPH: Cytochrome P450 reductase and prostaglandin H synthase mediate the formation of aristolochic acid-DNA adducts found in patients with urothelial cancer. Int J Cancer, 2005, 113: 189–197
Fernando R C, Schmeiser H H, Scherf H R, Wiessler M. Formation and persistence of specific purine DNA-adducts by 32P-postlabeling in target and non-target organs in rats treated with aristolochic acid I. IARC Sci Pub1, 1993, 124: 167–171
Schmeiser H H, Bieler C A, Wiessler M, van Ypersele de strihou C, anctiond Cosyns J P. Detection of DNA adducts formed by aristolochic acid in renal tissue from patients with Chinese herbs nephropathy. Cancer Res, 1996, 56: 2025–2028
Pfohl-Leszkowicz A, Tozlovanu M, Manderville R, Peraica M, Castegnaro M, Stefanovic V. New molecular and field evidence for the implication of mycotoxins but not aristolochic acid in human nephropathy and urinary tract tumor. Mol Nutr Food Res, 2007, 51: 1131–1146
Stiborova M, Sopko B, Hodek P, Frei E, Schmeiser H H, Hudecek J. The binding of aristolochic acid I to the active site of human cytochromes P450 1A1 and 1A2 explains their potential to reductively activate this human carcinogen. Cancer Lett, 2005, 229: 193–204
Stiborova M, Frei E, Wiessler M, Schmeiser H H. Human enzymes involved in the metabolic activation of carcinogenic aristolochic acids: Evidence for reductive activation by cytochromes P450 1A1 and 1A2. Chem Res Toxicol, 2001, 14: 1128–1137
Zeisig M, Moller L. 32P-HPLC suitable for characterization of DNA adducts formed in vitro by polycyclic aromatic hydrocarbons and derivatives. Carcinogenesis, 1995, 16: 1–9
Chan W, Lee K C, Liu N, Cai Z W. A sensitivity enhanced high-performance liquid chromatography fluorescence method for the detection of nephrotoxic and carcinogenic aristolochic acid in herbal medicines. J Chromatogr A, 2007, 1164: 113–119
Yuan J B, Liu Q, Zhu W F, Ding L, Tang F, Yao S Z. Simultaneous analysis of six aristolochic acids and five aristolactams in herbal plants and their preparations by high-performance liquid chromatogphy-diode array detection-fluorescence detection. J Chromatogr A, 2008, 1182: 85–92
Chaudhary A K, Nokubo M, Oglesby T D, Marnett L J, Blair I A. Characterization of endogenous DNA-adducts by liquid-hromagraphy electrospray-ionization tandem mass spectrometry. J Mass Spectrom, 1995, 30: 1157–1166
Paehler A, Richoz J, Soglia J, Vourous P. Analysis and quantification of DNA adducts of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in liver of rats by liquid chromatography/electrospray tandem mass spectrometry. Chem Res Toxicol, 2002, 15: 551–561
Qiu S X, Yang R Z, Gross M L. Synthesis and liquid chromatography/tandem mass spectrometric characterization of the adducts of bisphenol A o-quinone with glutathione and nucleotide monophosphates. Chem Res Toxicol, 2004, 17: 1038–1046
Tompkins E M, Jones D J L, Lamb J H, Marsden D A, Farmer P B, Brown K. Simultaneous detection of five different 2-ydroxyethyl-NA adducts formed by ethylene oxide exposure, using a high-erfornce liquid chromatography/electrospray ionization tandem mass spectrometry assay. Rapid Commun Mass Spectrom, 2008, 22: 19–28
Zhang F G, Stott W T, Clark A J, Schisler M R, Grundy J J, Gollapudi B B, Bartels M J. Quantitation of 8-hydroxydeoxyguanoisine in DNA by liquid chromatography/positive atmospheric pressure photoionization tandem mass spectrometry. Commun Mass Spectrom, 2007, 21: 3949–3955
Chan W, Cui L, Xu G W, Cai Z W. Study of the phase I and phase II metabolism of nephrotoxin aristolochic acid by liquid chromatography/tandem mass spectrometry. Commun Mass Spectrom, 2006, 20: 1755–1760
Chan W, Yue H, Poon W T, Chan Y W, Schmitz O J, Kwong D W J, Wong R N S, Cai Z W. Quantification of aristolochic acid-derived DNA adducts in rat kidney and liver by using liquid chromatography-electrospray ionization mass spectrometry. Mutat Res, 2008, 646: 17–24
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Research Grant Council, University Grants Committee of Hong Kong (Grant No. HKBU2459/06M), and the Health and Health Services Research Fund of Hong Kong (Grant No. 05060141)
Rights and permissions
About this article
Cite this article
Yue, H., Chan, W., Yu, K. et al. Recent progress in quantitative analysis of DNA adducts of nephrotoxin aristolochic acid. Sci. China Ser. B-Chem. 52, 1576–1582 (2009). https://doi.org/10.1007/s11426-009-0233-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-009-0233-6