Abstract
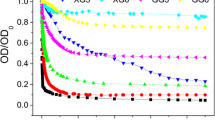
For in situ groundwater remediation, polyelectrolyte-modified nanoscale zerovalent iron particles (NZVIs) have to be delivered into the subsurface, where they degrade pollutants such as trichloroethylene (TCE). The effect of groundwater organic and ionic solutes on TCE dechlorination using polyelectrolyte-modified NZVIs is unexplored, but is required for an effective remediation design. This study evaluates the TCE dechlorination rate and reaction by-products using poly(aspartate) (PAP)-modified and bare NZVIs in groundwater samples from actual TCE-contaminated sites in Florida, South Carolina, and Michigan. The effects of groundwater solutes on short- and intermediate-term dechlorination rates were evaluated. An adsorbed PAP layer on the NZVIs appeared to limit the adverse effect of groundwater solutes on the TCE dechlorination rate in the first TCE dechlorination cycle (short-term effect). Presumably, the pre-adsorption of PAP “trains” and the Donnan potential in the adsorbed PAP layer prevented groundwater solutes from further blocking NZVI reactive sites, which appeared to substantially decrease the TCE dechlorination rate of bare NZVIs. In the second and third TCE dechlorination cycles (intermediate-term effect), TCE dechlorination rates using PAP-modified NZVIs increased substantially (~100 and 200%, respectively, from the rate of the first spike). The desorption of PAP from the surface of NZVIs over time due to salt-induced desorption is hypothesized to restore NZVI reactivity with TCE. This study suggests that NZVI surface modification with small, charged macromolecules, such as PAP, helps to restore NZVI reactivity due to gradual PAP desorption in groundwater.





Similar content being viewed by others
References
Agrawal A, Ferguson WJ, Gardner BO, Christ JA, Bandstra JZ, Tratnyek PG (2002) Effects of carbonate species on kinetics of dechlorination of 1,1,1-trichloroethane by zero-valent iron. Environ Sci Technol 36:4326–4333
Bennett P, He F, Zhao D, Aiken B, Feldman L (2010) In situ testing of metallic iron nanoparticle mobility and reactivity in a shallow granular aquifer. J Contam Hydrol 116:35–46
Bezbaruah AN, Shanbhogue SS, Simsek S, Khan E (2011) Encapsulation of iron nanoparticles in alginate biopolymer for trichloroethylene remediation. J Nanopart Res 13:6673–6681
Bishop EJ, Fowler DE, Skluzacek JM, Seibel E, Mallouk TE (2010) Anionic homopolymers efficiently target zerovalent iron particles to hydrophobic contaminants in sand columns. Environ Sci Technol 44:9069–9074
Burris DR, Delcomyn CA, Smith MH, Roberts AL (1996) Reductive dechlorination of tetrachloroethylene and trichloroethylene catalyzed by vitamin B12 in homogeneous and heterogeneous systems. Environ Sci Technol 30:3047–3052
de Carvalho SJ (2010) First-order–like transition in salt-induced macroion-polyelectrolyte desorption. EPL 92:18001
Devlin JF, Allin KO (2005) Major anion effects on the kinetics and reactivity of granular iron in glass-encased magnet batch reactor experiments. Environ Sci Technol 39:1868–1874
Edwards M, Benjamin MM, Ryan JN (1996) Role of organic acidity in sorption of natural organic matter (NOM) to oxide surfaces. Colloid Surface A 107:297–307
El-Naggar MM (2006) Effects of Cl−, NO3 −, and SO4 2− anions on the anodic behavior of carbon steel in deaerated 0.50 M NaHCO3 solutions. Appl Surf Sci 252:6179–6194
Evans DF, Wennerstrom H (1999) The colloidal domain; where physics, chemistry, biology, and technology meet. Wiley-VCH, New York
Golas PL, Louie S, Lowry GV, Matyjaszewski K, Tilton RD (2010) Comparative study of polymeric stabilizers for magnetite nanoparticles using ATRP. Langmuir 26:16890–16900
He F, Zhao D (2008) Hydrodechlorination of trichloroethene using stabilized Fe-Pd nanoparticles: reaction mechanism and effects of stabilizers, catalysts, and reaction conditions. Appl Catal B Environ 84:533–540
He F, Zhao D, Paul C (2010) Field assessment of carboxymethyl cellulose stabilized iron nanoparticles for in situ destruction of chlorinated solvents in source zones. Water Res 44:2360–2370
Henn KW, Waddill DW (2006) Utilization of nanoscale zero-valent iron for source remediation—a case study. Remediation J 16:57–77
Holmberg K, Jonsson B, Kronberg B, Lindman B (2003) Surfactants and polymers in aqueous solution, 2nd edn. John Wiley & Sons, Ltd., Chichester, West Sussex, England
Israelachvili JN (1992) Intermolecular and surface forces: with applications to colloidal and biological systems, 2nd edn. Academic Press, New York
Johnson TL, Fish W, Gorby YA, Tratnyek PG (1998) Degradation of carbon tetrachloride by iron metal: complexation effects on the oxide surface. J Contam Hydrol 29:379–398
Karn B, Kuiken T, Otto M (2009) Nanotechnology and in situ remediation: a review of the benefits and potential risks. Environ Health Perspect 117:1823–1831
Kepner RL Jr, Pratt JR (1994) Use of fluorochromes for direct enumeration of total bacteria in environmental samples: past and present. Microbiol Mol Biol R 58:603–615
Kim H-J, Phenrat T, Tilton RD, Lowry GV (2009) Fe0 nanoparticles remain mobile in porous media after aging due to slow desorption of polymeric surface modifiers. Environ Sci Technol 43:3824–3830
Kirschling TL, Gregory KB, Minkley EGJ, Lowry GV, Tilton RD (2010) Impact of nanoscale zero valent iron on geochemistry and microbial populations in trichloroethylene contaminated aquifer materials. Environ Sci Technol 44:3474–3480
Kirschling TL, Golas PL, Unrine JM, Matyjaszewski K, Gregory KB, Lowry GV, Tilton RD (2011) Microbial bioavailability of covalently bound polymer coatings on model engineered nanomaterials. Environ Sci Technol 45:5253–5259
Klausen J, Vikesland PJ, Kohn T, Burris DR, Ball WP, Roberts AL (2003) Longevity of granular iron in groundwater treatment processes: solution composition effects on reduction of organohalides and nitroaromatic compounds. Environ Sci Technol 37:1208–1218
Kocur CM et al (2014) Characterization of nZVI mobility in a field scale test. Environ Sci Technol 48:2862–2869
Kohn T, Livi KJT, Roberts AL, Vikesland PJ (2005) Longevity of granular iron in groundwater treatment processes: corrosion product development. Environ Sci Technol 39:2867–2879
Lim T-T, Zhu B-W (2008) Effects of anions on the kinetics and reactivity of nanoscale Pd/Fe in trichlorobenzene dechlorination. Chemosphere 73:1471–1477
Liu Y, Lowry GV (2006) Effect of particle age (Fe0 content) and solution pH on NZVI reactivity: H2 evolution and TCE dechlorination. Environ Sci Technol 40:6085–6090
Liu Y, Choi H, Dionysiou D, Lowry GV (2005a) Trichloroethene hydrodechlorination in water by highly disordered monometallic nanoiron. Chem Mater 17:5315–5322
Liu Y, Majetich SA, Tilton RD, Sholl DS, Lowry GV (2005b) TCE dechlorination rates, pathways, and efficiency of nanoscale iron particles with different properties. Environ Sci Technol 39:1338–1345
Liu Y, Phenrat T, Lowry GV (2007) Effect of TCE concentration and dissolved groundwater solutes on NZVI-promoted TCE dechlorination and H2 evolution. Environ Sci Technol 41:7881–7887
Lowry GV (2007) Nanomaterials for groundwater remediation. In: Wiesner MR, Bottero J-Y (eds) Environmental nanotechnology: applications and impacts of nanomaterials. McGraw-Hill, New York
Man X, Yang S, Yan D, Shi A-C (2008) Adsorption and depletion of polyelectrolytes in charged cylindrical system within self-consistent field theory. Macromolecules 41:5451–5456
Nurmi JT et al (2005) Characterization and properties of metallic iron nanoparticles: spectroscopy, electrochemistry, and kinetics. Environ Sci Technol 39:1221–1230
Ohshima H (1995) Electrophoresis of soft particles. Adv Colloid Interface Sci 62:189–235
Phenrat T, Saleh N, Sirk K, Tilton R, Lowry GV (2007) Aggregation and sedimentation of aqueous nanoscale zerovalent iron dispersions. Environ Sci Technol 41:284–290
Phenrat T, Saleh N, Sirk K, Kim H-J, Tilton RD, Lowry GV (2008) Stabilization of aqueous nanoscale zerovalent iron dispersions by anionic polyelectrolytes: adsorbed anionic polyelectrolyte layer properties and their effect on aggregation and sedimentation. J Nanopart Res 10:795–814
Phenrat T, Kim H-J, Fagerlund F, Illangasekare T, Tilton RD, Lowry GV (2009a) Particle size distribution, concentration, and magnetic attraction affect transport of polymer-modified Fe0 nanoparticles in sand columns. Environ Sci Technol 43:5079–5085
Phenrat T, Liu Y, Tilton R, Lowry GV (2009b) Adsorbed polyelectrolyte coatings decrease Fe0 nanoparticle reactivity with TCE in water: conceptual model and mechanisms. Environ Sci Technol 43:1507–1514
Phenrat T, Long TC, Lowry GV, Veronesi B (2009c) Partial oxidation (“aging”) and surface modification decrease the toxicity of nanosized zerovalent iron. Environ Sci Technol 43:195–200
Phenrat T, Cihan A, Kim H-J, Mital M, Illangasekare T, Lowry GV (2010) Transport and deposition of polymer-modified Fe0 nanoparticles in 2-D heterogeneous porous media: effects of particle concentration, Fe0 content, and coatings. Environ Sci Technol 44:9086–9093
Phenrat T, Fagerlund F, Illanagasekare T, Lowry GV, Tilton RD (2011) Polymer-modified Fe0 nanoparticles target entrapped NAPL in two dimensional porous media: effect of particle concentration, NAPL saturation, and injection strategy. Environ Sci Technol 45:6102–6109
Sakulchaicharoen N, O’Carroll DM, Herrera JE (2010) Enhanced stability and dechlorination activity of pre-synthesis stabilized nanoscale FePd particles. J Contam Hydrol 118:117–127
Saleh N, Phenrat T, Sirk K, Dufour B, Matyjaszewski K, Tilton RD, Lowry GV (2005) Adsorbed triblock copolymers deliver reactive iron nanoparticles to the oil/water interface. Nano Lett 12:2489–2494
Saleh N et al (2007) Surface modifications enhance nanoiron transport and NAPL targeting in saturated porous media. Environ Eng Sci 24:45–57
Saleh N, Kim H-J, Phenrat T, Matyjaszewski K, Tilton RD, Lowry GV (2008) Ionic strength and composition affect the mobility of surface-modified Fe0 nanoparticles in water-saturated sand columns. Environ Sci Technol 42:3349–3355
Sarathy V et al (2008) Aging of iron nanoparticles in aqueous solution: effects on structure and reactivity. J Phys Chem C 112:2286–2293
Scherer MM, Richter S, Valentine RL, Alvarez PJJ (2000) Chemistry and microbiology of permeable reactive barriers for in situ groundwater clean up. Crit Rev Microbiol 26:221–264
Song H, Carraway ER (2008) Catalytic hydrodechlorination of chlorinated ethenes by nanoscale zero-valent iron. Appl Catal B Environ 78:53–60
Su CM, Puls RW (2004) Nitrate reduction by zerovalent iron: effects of formate, oxalate, citrate, chloride, sulfate, borate, and phosphate. Environ Sci Technol 38:2715–2720
Tratnyek PG, Johnson RL (2006) Nanotechnologies for environmental cleanup. Nano Today 1:44–48
Van Nooten T, Springael D, Bastiaens L (2008) Positive impact of microorganisms on the performance of laboratory-scale permeable reactive iron barriers. Environ Sci Technol 42:1680–1686
Vecchia ED, Luna M, Sethi R (2009) Transport in porous media of highly concentrated iron micro- and nanoparticles in the presence of xanthan gum. Environ Sci Technol 43:8942–8947
Wang W, Zhou M (2010) Degradation of trichloroethylene using solvent-responsive polymer coated Fe nanoparticles. Colloid Surface A 369:232–239
Wang W, Zhou M, Jin Z, Li T (2010) Reactivity characteristics of poly(methyl methacrylate) coated nanoscale iron particles for trichloroethylene remediation. J Hazard Mater 173:724–730
Xiu ZM, Gregory KB, Lowry GV, Alvarez PJ (2010) Effect of bare and coated nanoscale zerovalent iron on tceA and vcrA gene expression in Dehalococcoides spp. Environ Sci Technol 44:7647–7651
Zhang W (2003) Nanoscale iron particles for environmental remediation: an overview. J Nanopart Res 5:323–332
Zhang W-X, Wang C-B, Lien H-L (1998) Treatment of chlorinated organic contaminants with nanoscale bimetallic particles. Catal Today 40:387–395
Acknowledgments
The authors are thankful for financial support from (1) the Thailand Research Fund (TRF) (MRG5680129), (2) the National Nanotechnology Centre (Thailand), a member of the National Science and Technology Development Agency, through grant number P-11-00989, (3) the National Research Council of Thailand (grant no. R2556B070 and R2555C010), and (4) the U.S. EPA (R830898 and R833326), NSF (BES-068646 and EF-0830093), and Department of Defense through the Strategic Environmental Research and Development Program (W912HQ-06-C-0038).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Additional file
Below is the link to the electronic supplementary material.
Additional file 1
Simplified TCE reduction pathways, Fe0 content determination, Ohshima’s soft particle analysis and mass balance of the dechlorination studies. This material is available free of charge via the Internet. (DOC 317 kb)
Rights and permissions
About this article
Cite this article
Phenrat, T., Schoenfelder, D., Kirschling, T.L. et al. Adsorbed poly(aspartate) coating limits the adverse effects of dissolved groundwater solutes on Fe0 nanoparticle reactivity with trichloroethylene. Environ Sci Pollut Res 25, 7157–7169 (2018). https://doi.org/10.1007/s11356-015-5092-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5092-4