Abstract
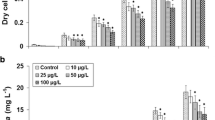
The effect of insecticides (acephate and imidacloprid) on a freshwater microalga Chlamydomonas mexicana was investigated with respect to photosynthetic pigments, carbohydrate and protein contents, fatty acids composition and induction of stress indicators including proline, superoxide dismutase (SOD) and catalase (CAT). C. mexicana was cultivated with 1, 5, 10, 15, 20 and 25 mg L−1 of acephate and imidacloprid. The microalga growth increased with increasing concentrations of both insecticides up to 15 mg L−1, beyond which the growth declined compared to control condition (without insecticides). C. mexicana cultivated with 15 mg L−1 of both insecticides for 12 days was used for further analysis. The accumulation of photosynthetic pigments (chlorophyll and carotenoids), carbohydrates and protein was decreased in the presence of both insecticides. Acephate and imidacloprid induced the activities of superoxide dismutase (SOD) and catalase (CAT) and increased the concentration of proline in the microalga, which play a defensive role against various environmental stresses. Fatty acid analysis revealed that the fraction of polyunsaturated fatty acids decreased on exposure to both insecticides. C. mexicana also promoted 25 and 21 % removal of acephate and imidacloprid, respectively. The biochemical changes in C. mexicana on exposure to acephate and imidacloprid indicate that the microalga undergoes an adaptive change in response to the insecticide-induced oxidative stress.




Similar content being viewed by others
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 5:121–126
Alia P, Saradhi P (1993) Suppression in mitochondrial electron transport is the prime cause behind stress induced proline accumulation. Biochem Biophys Res Commun 193:54–58. doi:10.1006/bbrc.1993.1589
Anhalt JC, Moorman TB, Koskinen WC (2007) Biodegradation of imidacloprid by an isolated soil microorganism. J Environ Sci Health Part B 42:509–514. doi:10.1080/03601230701391401
Babu GS, Hans RK, Singh J, Viswanathan PN, Joshi PC (2001) Effect of lindane on the growth and metabolic activities of cyanobacteria. Ecotoxicol Environ Saf 48:219–21. doi:10.1006/eesa.2000.2033
Bates LS, Wadern RP, Teare ID (1973) Rapid estimation of free proline for water stress determination. Plant Soil 39:205–207. doi:10.1007/BF00018060
Bischoff HW, Bold HC (1963) Phycological Studies IV. In: Some soil algae fromenchanted rock and related algal species. University of Texas Publication, Austin, pp 1-95
Choudhary M, Jetley UK, Khan MA, Zutshi S, Fatma T (2007) Effect of heavy metal stress on proline, malondialdehyde, and superoxide dismutase activity in the cyanobacterium Spirulina platensis-S5. Ecotoxicol Environ Saf 66:204–209. doi:10.1016/j.ecoenv.2006.02.002
Chuanjiang T, Dahui L, Xinzhong Z, Shanshan C, Lijuan F, Xiuying P, Jie S, Hui J, Chongjiu L, Jianzhong L (2010) Residue analysis of acephate and its metabolite methamidophos in open field and greenhouse pakchoi (Brassica campestrisL.) by gas chromatography-tandem mass spectrometry. Environ Monit Assess 165:685–692. doi:10.1007/s10661-009-0979-5
Cycon M, Wojcik M, Piotrowska-Seget Z (2009) Biodegradation of the organophosphorus insecticide diazinon by Serratia sp. and Pseudomonas sp. and their use in bioremediation of contaminated soil. Chemosphere 76:494–501. doi:10.1016/j.chemosphere.2009.03.023
Dhindsa RS, Dhindsa PP, Thorpe TA (1981) Leaf senescence: correlated with increased level of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101. doi:10.1093/jxb/32.1.93
Dosnon-Olette R, Trotel-Aziz P, Couderchet M, Eullaffroy P (2010) Fungicides and herbicide removal in Scenedesmus cell suspensions. Chemosphere 79:117–123. doi:10.1016/j.chemosphere.2010.02.005
DuBois M, Gilles K, Hamilton J, Rebers P, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356. doi:10.1021/ac60111a017
Elizabeth H. Harris (2008) The Chlamydomonas Sourcebook, Vol. 1: Introduction to Chlamydomonas and its laboratory use. Academic Press, Edition 2nd. ISBN-10: 0123708745
El-Salam Issa A, Adam MS, Fawzy MA (2013) Alterations in some metabolic activities of Scenedesmusquadricauda and Merismopediaglauca in response to glyphosate herbicide. J Biol Earth Sci 3(1):B17–B23
Fatma T, Khan MA, Choudhary M (2007) Impact of environmental pollution on cyanobacterialproline content. J Appl Phycol 19:625–629. doi:10.1007/s10811-007-9195-2
Felsot A, Maddox JV, Bruce W (1989) Enhanced microbial degradation of carbofuran in soils with histories of carbofuran use. Bull Environ Contam Toxicol 26:781–788. doi:10.1007/BF01622171
Galhano V, Laranjo JG, Peixoto F (2011a) Exposure of the cyanobacterium Nostocmuscorum from Portuguese rice fields to Molinate (Ordram®): Effects on the antioxidant system and fatty acid profile. Aquat Toxicol 101:367–376. doi:10.1016/j.aquatox.2010.11.011
Galhano V, Santos H, Oliveira MM, Laranjo JG, Peixoto F (2011b) Changes in fatty acid profile and antioxidant systems in a Nostocmuscorum strain exposed to the herbicide bentazon. Process Biochem 46:2152–2162. doi:10.1016/j.procbio.2011.08.015
Gibbons D, Morrissey C, Mineau P (2014) A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environ Sci Pollut Res. doi:10.1007/s11356-014-3180-5
Hellebust JA, Craige JS (1978) Handbook of physiological and biochemical methods. Cambridge university press, Cambridge, pp 64–70
Hirooka T, Nagase H, Uchida K, Hiroshige Y, Ehara Y, Nishikawa J, Nishihara T, Miyamoto K, Hirata Z (2005) Biodegradation of bisphenol A and disappearance of its estrogenic activity by the green alga Chlorella fuscavar. vacuolata. Environ Toxicol Chem 24:1896–1901. doi:10.1897/04-259R.1
Imlay JA, Chin SM, Linn S (1998) Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science 240:640–642. doi:10.1126/science.2834821
Ingram CW, Coyne MS, Williams DW (2005) Effects of commercial diazinon and imidacloprid on microbial urease activity in soil and sod. J Environ Qual 34:1573–1580. doi:10.2134/jeq2004.0433
Janknegt PJ, Rijstenbil JW, Van de Poll WH, Gechev TS, Buma AGJ (2007) A comparison of quantitative and qualitative Superoxide dismutase assays for application to low temperature microalgae. J Photochem Photobiol B Biol 87:218–226. doi:10.1016/j.jphotobiol.2007.04.002
Jemec A, Tisler T, Drobne D, Sepcic K, Fournier D, Trebse P (2007) Comparative toxicity of imidacloprid, of its commercial liquid formulation and of diazinon to a non-target arthropod, the microcrustaceanDaphnia magna. Chemosphere 68:1408–1418. doi:10.1016/j.chemosphere.2007.04.015
Ji MK, Kabra AN, Choi J, Hwang JH, Kim JR, Abou-Shanab RAI, Oh YK, Jeon BH (2014) Biodegradation of bisphenol A by the freshwater microalgae Chlamydomonasmexicana and Chlorellavulgaris. Ecol Eng 73:260–269. doi:10.1016/j.ecoleng.2014.09.070
Kabra AN, Ji MK, Choi J, Kim JR, Govindwar SP, Jeon BH (2014) Toxicity of atrazine and its bioaccumulation and biodegradation in a green microalga, Chlamydomonasmexicana. Environ Sci Pollut Res 21:12270–12278. doi:10.1007/s11356-014-3157-4
Kumar MS, Praveenkumar R, Jeon BH, Thajuddin N (2014) Chlorpyrifos induced changes in the antioxidants and fatty acid compositions of Chroococcusturgidus NTMS12. Lett Appl Microbiol 59(5):535–541. doi:10.1111/lam.12311
Kumar MS, Praveenkumar R, Ilavarasi A, Rajeshwari K, Thajuddin N (2013) Biochemical changes of fresh water cyanobacteria Dolichospermumflos-aquae NTMS07 to chromium-induced stress with special reference to antioxidant enzymes and cellular fatty acids. Bull Environ Contam Toxicol 90:730–735. doi:10.1007/s00128-013-0984-9
Kumar MS, Praveenkumar R, Ilavarasi A, Rajeshwari K, Thajuddin N (2012a) Oxidative stress response and fatty acid changes associated with bioaccumulation of chromium by a fresh water cyanobacterium Chroococcus sp. Biotechnol Lett 34:247–251. doi:10.1007/s10529-011-0771-9
Kumar N, Bora A, Kumar R, Amb MK (2012b) Differential Effects of Agricultural Pesticides Endosulfan and Tebuconazole on Photosynthetic pigments, Metabolism and Assimilating Enzymes of Three Heterotrophic, Filamentous Cyanobacteria. J Biol Environ Sci 6:67–75
Kumar S, Habib K, Fatma T (2008) Endosulfan induced biochemical changes in nitrogen-fixing cyanobacteria. Sci Total Environ 403:130–138. doi:10.1016/j.scitotenv.2008.05.026
Kwon CS, Penner D (1995) The interaction of insecticides with herbicide activity. Weed Technol 9:119–124
Leitao MAS, Cardozo KHM, Pinto E, Colepicolo P (2003) PCB-Induced oxidative stress in the unicellular marine dinoflagellateLingulodiniumpolyedrum. Arch Environ Contam Toxicol 45:59–65. doi:10.1007/s00244-002-0208-5
Lepage G, Roy CC (1984) Improved recovery of fatty acid through direct transesterification without prior extraction or purification. J Lipid Res 25:1391–1396
Lin X, Xu X, Yang C, Zhao Y, Feng Z, Dong Y (2009) Activities of antioxidant enzymes in three bacteria exposed to bensulfuron-methyl. Ecotoxicol Environ Saf 72:1899–1904. doi:10.1016/j.ecoenv.2009.04.016
Littlefield-Wyer JG, Brooks P, Katouli A (2008) Application of biochemical fingerprinting and fatty acid methyl ester profiling to assess the effect of the pesticide Atradex on aquatic microbial communities. Environ Pollut 153:393–400. doi:10.1016/j.envpol.2007.08.016
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Follin. phenol reagent. J Biol Chem 193(1):265–275
Mohapatra S, Ahuja AK, Deepa M, Sharma D (2011) Residues of acephate and its metabolite methamidophos in/on mango fruit (Mangiferaindica L.). Bull Environ Contam Toxicol 86:101–104. doi:10.1007/s00128-010-0154-2
Motulsky HJ (2007) Prism 5 statistics guide. GraphPad Software Inc, San Diego CA
Newsted JL (2004) Effect of light, temperature, and pH on the accumulation of phenol by Selenastrumcapricornutum, a green alga. Ecotoxicol Environ Saf 59:237–243. doi:10.1016/j.ecoenv.2003.07.009
Okamoto OKE, Pinto E, Latorre LR, Bechara EJH, Colepicolo P (2001) Antioxidant modulation in response to metal-induced oxidative stress in algal chloroplasts. Arch Environ Contam Toxicol 40:18–24. doi:10.1007/s002440010144
Olga B, Eija V, Kurt VF (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–94. doi:10.1093/aob/mcf118
Porra RJ, Thompson WA, Kriedmann PE (1989) Determination of accurate extinction co-efficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394. doi:10.1016/S0005-2728(89)80347-0
Prasad SM, Kumar D, Zeeshan M (2005) Growth photosynthesis, active oxygen species and antioxidants responses of paddy field cyanobacterium Plectonemaboryanumto endosulfan stress. J Gen Appl Microbiol 51:115–123. doi:10.2323/jgam.51.115
Ramu S, Seetharaman B (2014) Biodegradation of acephate and methamidophos by a soil bacterium Pseudomonas aeruginosa strain Is-6. J Environ Sci Health Part B49:23–34. doi:10.1080/03601234.2013.836868
Rodger PA, Simpson I, Oficial R, Ardales S, Jimenez R (1994) Effects of pesticides on soil and water microflora and mesofauna in wetland rice fields: a summary of current knowledge and extrapolation to temperate environments. Aust J Exp Agric 34:1057–1068. doi:10.1071/EA9941057
Saroja G, Bose S (1982) Effect of methylparathion on the growth, cell size, pigment and protein content of Chlorellaprotothecoides. Environ Pollut 27:297–308. doi:10.1016/0143-1471(82)90158-1
Satish N, Tiwari GL (2000) Pesticide tolerance in Nostoclinckia in relation to the growth and nitrogen fixation. Proc Natl Acad Sci India 70:319–323
Singh BK, Walker A, Wright DJ (2006) Bioremedial potential of fenamiphos and chlorpyrifos degrading isolates: Influence of different environmental conditions. Soil Biol Biochem. doi:10.1016/j.soilbio.2006.04.019
Singh SC, Sinha RP, Hader DP (2002) Role of lipids and fatty acids in stress tolerance in cyanobacteria. Acta Protozool 41:297–308
Srivastava AK, Bhargava P, Rai LC (2005) Salinity and copper-induced oxidative damage and changes in the antioxidative defence systems of Anabaena doliolum. World J Microbiol Biotechnol 21:1291–1298. doi:10.1007/s11274-005-2442-2
Vandana S, Basu DB, Asit KC (2001) Lipid peroxidation, free radical scavenging enzymes, and glutathione redox system in blood of rats exposed to propoxur. Pestic Biochem Physiol 71:133–139. doi:10.1006/pest.2001.2571
Wiktelius E, Stenberg G (2007) Novel class of glutathione transferases from cyanobacteria exhibit high catalytic activities towards naturally occurring isothiocyanates. Biochem J406:115–123. doi:10.1042/BJ20070328
Wright SJL, Stantharpe AF, Downs JD (1997) Interaction of the herbicide propanil and a metabolite. 3,4-dichloromiline, with blue green algae. Acta Pathol Acad Sci Hung 12:51–60
Zarger MY, Dar GH (1990) Effectof benthiocarb and butachlor on growth and nitrogen fixation by cyanobacteria45:232–234. Bull Environ Contam Toxicol. doi:10.1007/BF01700189
Acknowledgments
This work was supported by the Mid-career Researcher Program (National Research Foundation of Korea, 2013069183). The financial support from the Hanyang University, South Korea, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Cinta Porte
Rights and permissions
About this article
Cite this article
Kumar, M.S., Kabra, A.N., Min, B. et al. Insecticides induced biochemical changes in freshwater microalga Chlamydomonas mexicana . Environ Sci Pollut Res 23, 1091–1099 (2016). https://doi.org/10.1007/s11356-015-4681-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4681-6