Abstract
Introduction
Older patients are more likely to acquire and die from acute respiratory distress syndrome (ARDS) and muscle weakness may be more clinically significant in older persons. Recent data implicate muscle ring finger protein 1 (MuRF1) in lung injury-induced skeletal muscle atrophy in young mice and identify an alternative role for MuRF1 in cardiac metabolism regulation through inhibition of fatty acid oxidation.
Objectives
To develop a model of lung injury-induced muscle wasting in old mice and to evaluate the skeletal muscle metabolomic profile of adult and old acute lung injury (ALI) mice.
Methods
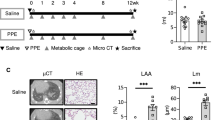
Young (2 month), adult (6 month) and old (20 month) male C57Bl6 J mice underwent Sham (intratracheal H2O) or ALI [intratracheal E. coli lipopolysaccharide (i.t. LPS)] conditions and muscle functional testing. Metabolomic analysis on gastrocnemius muscle was performed using gas chromatography-mass spectrometry (GC–MS).
Results
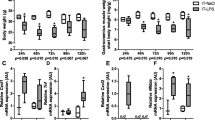
Old ALI mice had increased mortality and failed to recover skeletal muscle function compared to adult ALI mice. Muscle MuRF1 expression was increased in old ALI mice at day 3. Non-targeted muscle metabolomics revealed alterations in amino acid biosynthesis and fatty acid metabolism in old ALI mice. Targeted metabolomics of fatty acid intermediates (acyl-carnitines) and amino acids revealed a reduction in long chain acyl-carnitines in old ALI mice.
Conclusion
This study demonstrates age-associated susceptibility to ALI-induced muscle wasting which parallels a metabolomic profile suggestive of altered muscle fatty acid metabolism. MuRF1 activation may contribute to both atrophy and impaired fatty acid oxidation, which may synergistically impair muscle function in old ALI mice.






Similar content being viewed by others
Abbreviations
- ALI:
-
Acute lung injury
- ARDS:
-
Acute respiratory distress syndrome
- LPS:
-
Lipopolysaccharide
- GAS:
-
Gastrocnemius
- EDL:
-
Extensor digitorum longus
- SOL:
-
Soleus
- TA:
-
Tibialis anterior
References
Ali, N. A., et al. (2008). Acquired weakness, handgrip strength, and mortality in critically ill patients. American Journal of Respiratory and Critical Care Medicine, 178, 261–268. doi:10.1164/rccm.200712-1829OC.
Amato, M. B., et al. (2015). Driving pressure and survival in the acute respiratory distress syndrome. New England Journal of Medicine, 372, 747–755. doi:10.1056/NEJMsa1410639.
An, J., et al. (2004). Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nature Medicine, 10, 268–274. doi:10.1038/nm995.
Baehr, L. M., et al. (2016). Age-related deficits in skeletal muscle recovery following disuse are associated with neuromuscular junction instability and ER stress, not impaired protein synthesis. Aging (Albany NY), 8, 127–146.
Banerjee, R., et al. (2015). Non-targeted metabolomics of double-mutant cardiomyocytes reveals a novel role for SWI/SNF complexes in metabolic homeostasis. Metabolomics, 11, 1287–1301. doi:10.1007/s11306-015-0786-7.
Bienvenu, O. J., et al. (2012). Depressive symptoms and impaired physical function after acute lung injury: a 2-year longitudinal study. American Journal of Respiratory and Critical Care Medicine, 185, 517–524. doi:10.1164/rccm.201103-0503OC.
Bodine, S. C., & Baehr, L. M. (2014). Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab, 307, E469–E484. doi:10.1152/ajpendo.00204.2014.
Bodine, S. C., et al. (2001). Identification of ubiquitin ligases required for skeletal muscle atrophy. Science, 294, 1704–1708. doi:10.1126/science.1065874.
Chatfield, K. C., et al. (2015). Mitochondrial energy failure in HSD10 disease is due to defective mtDNA transcript processing. Mitochondrion, 21, 1–10. doi:10.1016/j.mito.2014.12.005.
Choi, S. J., et al. (2016). Intramyocellular lipid and impaired myofiber contraction in normal weight and obese older adults. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 71, 557–564. doi:10.1093/gerona/glv169.
Consitt, L. A., Bell, J. A., & Houmard, J. A. (2009). Intramuscular lipid metabolism, insulin action, and obesity. IUBMB Life,. doi:10.1002/iub.142.
Cruz-Jentoft, A. J., et al. (2010). Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age and Ageing, 39, 412–423. doi:10.1093/ageing/afq034.
Der-Torossian, H., et al. (2013). Cancer cachexia’s metabolic signature in a murine model confirms a distinct entity. Metabolomics 7, Metabolomics,. doi:10.1007/s11306-012-0485-6.
Duval, C., Camara, Y., Hondares, E., Sibille, B., & Villarroya, F. (2007). Overexpression of mitochondrial uncoupling protein-3 does not decrease production of the reactive oxygen species, elevated by palmitate in skeletal muscle cells. FEBS Letters, 581, 955–961. doi:10.1016/j.febslet.2007.01.085.
Ehlenbach, W. J., Larson, E. B., Randall Curtis, J., & Hough, C. L. (2015). Physical function and disability after acute care and critical illness hospitalizations in a prospective cohort of older adults. Journal of the American Geriatrics Society,. doi:10.1111/jgs.13663.
Ely, E. W., Wheeler, A. P., Thompson, B. T., Ancukiewicz, M., Steinberg, K. P., & Bernard, G. R. (2002). Recovery rate and prognosis in older persons who develop acute lung injury and the acute respiratory distress syndrome. Annals of Internal Medicine, 136, 25–36.
Ewaschuk, J. B., Almasud, A., & Mazurak, V. C. (2014). Role of n-3 fatty acids in muscle loss and myosteatosis. Applied Physiology, Nutrition and Metabolism, 39, 654–662. doi:10.1139/apnm-2013-0423.
Ferrante, L. E., Pisani, M. A., Murphy, T. E., Gahbauer, E. A., Leo-Summers, L. S., & Gill, T. M. (2015). Functional trajectories among older persons before and after critical illness. JAMA Intern Med, 175, 523–529. doi:10.1001/jamainternmed.2014.7889.
Ferrante, L. E., Pisani, M. A., Murphy, T. E., Gahbauer, E. A., Leo-Summers, L. S., & Gill, T. M. (2016). Factors associated with functional recovery among older ICU survivors. American Journal of Respiratory and Critical Care Medicine,. doi:10.1164/rccm.201506-1256OC.
Files, D. C., Sanchez, M. A., & Morris, P. E. (2015a). A conceptual framework: the early and late phases of skeletal muscle dysfunction in the acute respiratory distress syndrome. Critical Care, 19, 266. doi:10.1186/s13054-015-0979-5.
Files, D. C., et al. (2012). A critical role for muscle ring finger-1 in acute lung injury-associated skeletal muscle wasting. American Journal of Respiratory and Critical Care Medicine, 185, 825–834. doi:10.1164/rccm.201106-1150OC.
Files, D. C., et al. (2015b). Therapeutic exercise attenuates neutrophilic lung injury and skeletal muscle wasting. Sci Transl Med, 7(278), ra32. doi:10.1126/scitranslmed.3010283.
Force, A. D. T., et al. (2012). Acute respiratory distress syndrome: the Berlin definition. JAMA, 307, 2526–2533. doi:10.1001/jama.2012.5669.
Fukawa, T., et al. (2016). Excessive fatty acid oxidation induces muscle atrophy in cancer cachexia. Nature Medicine,. doi:10.1038/nm.4093.
Garvey, S. M., et al. (2014). Metabolomic profiling reveals severe skeletal muscle group-specific perturbations of metabolism in aged FBN rats. Biogerontology, 15, 217–232. doi:10.1007/s10522-014-9492-5.
Goodpaster, B. H., et al. (2006). The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 61, 1059–1064.
Hermans, G., et al. (2014). Acute outcomes and 1-year mortality of intensive care unit-acquired weakness. A cohort study and propensity-matched analysis. American Journal of Respiratory and Critical Care Medicine, 190, 410–420. doi:10.1164/rccm.201312-2257OC.
Herridge, M. S., et al. (2011). Functional disability 5 years after acute respiratory distress syndrome. New England Journal of Medicine, 364, 1293–1304. doi:10.1056/NEJMoa1011802.
Jensen, M. V., et al. (2006). Compensatory responses to pyruvate carboxylase suppression in islet beta-cells. Preservation of glucose-stimulated insulin secretion. Journal of Biological Chemistry, 281, 22342–22351. doi:10.1074/jbc.M604350200.
Kamolrat, T., & Gray, S. R. (2013). The effect of eicosapentaenoic and docosahexaenoic acid on protein synthesis and breakdown in murine C2C12 myotubes. Biochem Biophys Res Commun, 432, 593–598. doi:10.1016/j.bbrc.2013.02.041.
Kumar, K. V., Rao, S. M., Gayani, R., Mohan, I. K., & Naidu, M. U. (2000). Oxidant stress and essential fatty acids in patients with risk and established ARDS. Clinica Chimica Acta, 298, 111–120.
Langen, R. C., et al. (2012). NF-κB activation is required for the transition of pulmonary inflammation to muscle atrophy. American Journal of Respiratory Cell and Molecular Biology, 47, 288–297. doi:10.1165/rcmb.2011-0119OC.
Larsen, A. E., & Crowe, T. C. (2009). Effects of conjugated linoleic acid on myogenic and inflammatory responses in a human primary muscle and tumor coculture model. Nutrition and Cancer, 61, 687–695. doi:10.1080/01635580902898750.
Li, L. O., et al. (2015). Compartmentalized acyl-CoA metabolism in skeletal muscle regulates systemic glucose homeostasis. Diabetes, 64, 23–35. doi:10.2337/db13-1070.
Manzano, F., et al. (2005). Incidence of acute respiratory distress syndrome and its relation to age. Journal of Critical Care, 20, 274–280. doi:10.1016/j.jcrc.2005.05.008.
Matthay, M. A., Ware, L. B., & Zimmerman, G. A. (2012). The acute respiratory distress syndrome. J Clin Invest, 122, 2731–2740. doi:10.1172/JCI60331.
Metter, E. J., Lynch, N., Conwit, R., Lindle, R., Tobin, J., & Hurley, B. (1999). Muscle quality and age: cross-sectional and longitudinal comparisons. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 54, B207–B218.
Morley, J. E., Thomas, D. R., & Wilson, M.-M. G. (2006). Cachexia: pathophysiology and clinical relevance. American Journal of Clinical Nutrition, 83, 735–743.
O’Connell, T. M., et al. (2008). Metabolomic analysis of cancer cachexia reveals distinct lipid and glucose alterations. Metabolomics, 4, 216–225. doi:10.1007/s11306-008-0113-7.
Pariza, M. W., Park, Y., & Cook, M. E. (1999). Conjugated linoleic acid and the control of cancer and obesity. Toxicological Sciences, 52, 107–110.
Picard, M., et al. (2012). Mitochondrial dysfunction and lipid accumulation in the human diaphragm during mechanical ventilation. American Journal of Respiratory and Critical Care Medicine, 186, 1140–1149. doi:10.1164/rccm.201206-0982OC.
Prabha, P. S., Das, U. N., Ramesh, G., Kumar, K. V., & Kamalakar, V. (1991). Free radical generation, lipid peroxidation and essential fatty acids in patients with septicemia. Prostaglandins Leukotrienes and Essential Fatty Acids, 42, 61–65.
Prows, D. R., Gibbons, W. J, Jr., Smith, J. J., Pilipenko, V., & Martin, L. J. (2015). Age and sex of mice markedly affect survival times associated with hyperoxic acute lung injury. PLoS One, 10, e0130936. doi:10.1371/journal.pone.0130936.
Pugh, T. D., et al. (2013). A shift in energy metabolism anticipates the onset of sarcopenia in rhesus monkeys. Aging Cell, 12, 672–681. doi:10.1111/acel.12091.
Quinlan, G. J., Lamb, N. J., Evans, T. W., & Gutteridge, J. M. (1996). Plasma fatty acid changes and increased lipid peroxidation in patients with adult respiratory distress syndrome. Critical Care Medicine, 24, 241–246.
Rodriguez, J. E., et al. (2015). The ubiquitin ligase MuRF1 regulates PPARα activity in the heart by enhancing nuclear export via monoubiquitination. Molecular and Cellular Endocrinology, 413, 36–48. doi:10.1016/j.mce.2015.06.008.
Rubenfeld, G. D., et al. (2005). Incidence and outcomes of acute lung injury. New England Journal of Medicine, 353, 1685–1693. doi:10.1056/NEJMoa050333.
Smith, H. J., Lorite, M. J., & Tisdale, M. J. (1999). Effect of a cancer cachectic factor on protein synthesis/degradation in murine C2C12 myoblasts: modulation by eicosapentaenoic acid. Cancer Research, 59, 5507–5513.
Tarnopolsky, M. A., & Safdar, A. (2008). The potential benefits of creatine and conjugated linoleic acid as adjuncts to resistance training in older adults. Applied Physiology, Nutrition and Metabolism, 33, 213–227. doi:10.1139/H07-142.
Tian, M., Kliewer, K. L., Asp, M. L., Stout, M. B., & Belury, M. A. (2011). c9t11-Conjugated linoleic acid-rich oil fails to attenuate wasting in colon-26 tumor-induced late-stage cancer cachexia in male CD2F1 mice. Molecular Nutrition & Food Research, 55, 268–277. doi:10.1002/mnfr.201000176.
Wu, J. Y., et al. (2004). ENU mutagenesis identifies mice with mitochondrial branched-chain aminotransferase deficiency resembling human maple syrup urine disease. J Clin Invest, 113, 434–440. doi:10.1172/JCI19574.
Xia, J., Psychogios, N., Young, N., & Wishart, D. S. (2009). MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Research, 37, W652–W660. doi:10.1093/nar/gkp356.
Xia, J., Sinelnikov, I. V., Han, B., & Wishart, D. S. (2015). MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic Acids Research, 43, W251–W257. doi:10.1093/nar/gkv380.
Xia, J., Sinelnikov, I. V., & Wishart, D. S. (2011). MetATT: a web-based metabolomics tool for analyzing time-series and two-factor datasets. Bioinformatics, 27, 2455–2456. doi:10.1093/bioinformatics/btr392.
Zulet, M. A., Marti, A., Parra, M. D., & Martinez, J. A. (2005). Inflammation and conjugated linoleic acid: mechanisms of action and implications for human health. J Physiol Biochem, 61, 483–494.
Acknowledgments
This work was supported by the National Institutes of Health (R01HL104129 to M.W. and R01AG13934 to O.D.), the Leducq Foundation Transatlantic Networks of Excellence (to M.W.), the Claude D. Pepper Older Americans Independence Center (P30AG21332 to D.C.F and O.D.), and the American Thoracic Society Foundation (D.C.F.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
D. C. Files and A. Ilaiwy have contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11306_2016_1079_MOESM1_ESM.pdf
Supplemental Figure 1. Enrichment analysis of significant non-targeted metabolomics analysis of adult (6 month) gastrocnemius tissue 10 days post ALI or Sham. Enrichment by A) Disease-associated metabolite sets in the blood. B) Disease associated metabolite sets in CSF, and C) Location-Based Metabolite sets determined from VIP significant and t-test significant metabolites identified. Supplemental Figure 2. Enrichment analysis of significant non-targeted metabolomics analysis of old (20 month) gastrocnemius tissue 10 days post ALI or Sham Enrichment by A) Disease-associated metabolite sets in the blood. B) Disease associated metabolite sets in CSF, and C) Location-Based Metabolite sets determined from VIP significant and t-test significant metabolites identified. Supplemental Figure 3. AGE-dependent and ALI-dependent metabolites found by non-targeted metabolomics analysis. A) Significant AGE-dependent metabolites (including metabolites altered in all old vs sham, but not in adult ALI) and ALI-Dependent metabolites (Significantly different in all ALI, but not in Old or Adult Sham). B) Normalized peak values of the AGE-dependent docosahexanoic acid (DHA) among the four groups studied. C) Normalized peak values of the ALI-dependent linoleic acid among the four groups studied. No significant alterations in linoleic acid levels were detected between adult and old Sham mice by post hoc test. *p<0.05 vs. ALI adult, ^p<0.05 vs Sham old. #p<0.05 vs Sham adult. +p<0.05 vs Sham adult. N=10/group. Supplemental Figure 4. Significantly altered acyl-carnitine species in old gastrocnemius tissue 10 days post-ALI demonstrating the specific inhibition of long chain acyl-carnitines. X axis represents fatty acid chain length, measured by number of carbons in their structure. Acylcarnitines above the dotted green line had higher concentration in ALI mice compared to Sham. Acylcarnitines with 2-5 carbons in their structure were considered short. Acylcarnitines with 6-12 carbons were considered medium, and acylcarntines with 14-22 carbons were considered long. A Student’s T-test was used to determine significance between groups, with significance defined as p<0.05. Supplemental Figure 5. Targeted acyl-carnitine profile of old ALI muscle reveals significant decreases in long chain acyl carnitine (C14-C22), paralleling the phenotype seen in cardiac muscle with increased MuRF1 expression. A) Heat map of the One-Way ANOVA significant acyl-carnitines, determined by Fisher LSD post hoc test results. Fisher LSD post hoc comparisons were made to Sham adult muscle. Mean of each metabolite level in control groups was standardized to 1, and metabolite levels in experimental groups were then normalized to their control mean. Metabolites identified were later used to plot acyl carnitine concentration curve in each model. B) Box and whisker plot of total long chain acyl-carnitines using normalized concentrations measured by targeted metabolomics among eight groups of comparison. *p<0.05 MuRF1-/- vs. ALI adult, +p<0.05 MuRF1-/- vs. ALI old, $p<0.05 MuRF1-/- vs MuRF1 Tg+, #p<0.05 ALI old vs Sham old, ^p<0.05 ALI old vs Sham adult. C) Fold change of acyl-carnitine concentrations in mice normalized to their controls. X axis represents fatty acid chain length, measured by number of carbons in their structure. Y axis represents acyl-carnitine fold change. Each exponential line represents acyl-carnitine fold change in its specific group, with identified acyl-carnitines plotted in panel. Only significantly altered acyl-carnitines in Fisher LSD post hoc results among all groups were dot-plotted in this figure (p<0.05). D) ANOVA significant acyl-carnitine species. Long chain acyl-carnitines defined as C14-C22; Medium chain acyl-carnitines defined as C6-C13; Short chain acyl-carnitines C2-C5. N=10/adult Sham, adult ALI, old Sham, old ALI, N=3/WildtypeMuRF1Tg+, MuRF1 Tg+, N=4/MuRF1+/+, MuRF1-/-. Significance was defined as p<0.05. Supplemental Figure 6. Pathway enrichment analysis of ALI-dependent metabolites, determined by non-targeted metabolomics. a-c indicates top pathways identified, along with the specific significant metabolites found that placed it in this category. ALI dependent metabolites determined by Fisher LSD post hoc results were used in the pathway enrichment analysis. Of note, pathway enrichment analysis of Age dependent metabolites (urea, docosahexanoic acid) was also performed, but no altered pathways were detected. (PDF 1553 kb)
Rights and permissions
About this article
Cite this article
Files, D.C., Ilaiwy, A., Parry, T.L. et al. Lung injury-induced skeletal muscle wasting in aged mice is linked to alterations in long chain fatty acid metabolism. Metabolomics 12, 134 (2016). https://doi.org/10.1007/s11306-016-1079-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-016-1079-5