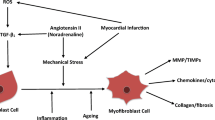
Abstract
Adenosine levels increase in ischemic hearts and contribute to the modulation of that pathological environment. We previously showed that A2B adenosine receptors on mouse cardiac Sca1+CD31− mesenchymal stromal cells upregulate secretion of paracrine factors that may contribute to the improvement in cardiac recovery seen when these cells are transplanted in infarcted hearts. In this study, we tested the hypothesis that A2B receptor signaling regulates the transition of Sca1+CD31− cells, which occurs after myocardial injury, into a myofibroblast phenotype that promotes myocardial repair and remodeling. In vitro, TGFβ1 induced the expression of the myofibroblast marker α-smooth muscle actin (αSMA) and increased collagen I generation in Sca1+CD31− cells. Stimulation of A2B receptors attenuated TGFβ1-induced collagen I secretion but had no effect on αSMA expression. In vivo, myocardial infarction resulted in a rapid increase in the numbers of αSMA-positive cardiac stromal cells by day 5 followed by a gradual decline. Genetic deletion of A2B receptors had no effect on the initial accumulation of αSMA-expressing stromal cells but hastened their subsequent decline; the numbers of αSMA-positive cells including Sca1+CD31− cells remained significantly higher in wild type compared with A2B knockout hearts. Thus, our study revealed a significant contribution of cardiac Sca1+CD31− cells to the accumulation of αSMA-expressing cells after infarction and implicated A2B receptor signaling in regulation of myocardial repair and remodeling by delaying deactivation of these cells. It is plausible that this phenomenon may contribute to the beneficial effects of transplantation of these cells to the injured heart.






Similar content being viewed by others
References
Rossini A, Frati C, Lagrasta C, Graiani G, Scopece A, Cavalli S, Musso E, Baccarin M, Di SM, Fagnoni F, Germani A, Quaini E, Mayr M, Xu Q, Barbuti A, DiFrancesco D, Pompilio G, Quaini F, Gaetano C, Capogrossi MC (2011) Human cardiac and bone marrow stromal cells exhibit distinctive properties related to their origin. Cardiovasc Res 89:650–660
Li TS, Cheng K, Malliaras K, Smith RR, Zhang Y, Sun B, Matsushita N, Blusztajn A, Terrovitis J, Kusuoka H, Marban L, Marban E (2012) Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol 59:942–953
Huang C, Gu H, Yu Q, Manukyan MC, Poynter JA, Wang M (2011) Sca-1+ cardiac stem cells mediate acute cardioprotection via paracrine factor SDF-1 following myocardial ischemia/reperfusion. PLoS One 6:e29246
Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H, Sano M, Toko H, Akazawa H, Sato T, Nakaya H, Kasanuki H, Komuro I (2004) Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem 279:11384–11391
Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS, Liao R (2005) CD31− but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res 97:52–61
Wang X, Hu Q, Nakamura Y, Lee J, Zhang G, From AH, Zhang J (2006) The role of the sca-1+/CD31− cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells 24:1779–1788
Mohri T, Fujio Y, Obana M, Iwakura T, Matsuda K, Maeda M, Azuma J (2009) Signals through glycoprotein 130 regulate the endothelial differentiation of cardiac stem cells. Arterioscler Thromb Vasc Biol 29:754–760
Liang SX, Tan TY, Gaudry L, Chong B (2010) Differentiation and migration of Sca1+/CD31− cardiac side population cells in a murine myocardial ischemic model. Int J Cardiol 138:40–49
Matsuura K, Honda A, Nagai T, Fukushima N, Iwanaga K, Tokunaga M, Shimizu T, Okano T, Kasanuki H, Hagiwara N, Komuro I (2009) Transplantation of cardiac progenitor cells ameliorates cardiac dysfunction after myocardial infarction in mice. J Clin Invest 119:2204–2217
Ryzhov S, Goldstein AE, Novitskiy SV, Blackburn MR, Biaggioni I, Feoktistov I (2012) Role of A2B adenosine receptors in regulation of paracrine functions of stem cell antigen 1-positive cardiac stromal cells. J Pharmacol Exp Ther 341:764–774
Ryzhov S, Zhang Q, Biaggioni I, Feoktistov I (2013) Adenosine A2B receptors on cardiac stem cell antigen (Sca)-1-positive stromal cells play a protective role in myocardial infarction. Am J Pathol 183:665–672
Tateishi K, Ashihara E, Takehara N, Nomura T, Honsho S, Nakagami T, Morikawa S, Takahashi T, Ueyama T, Matsubara H, Oh H (2007) Clonally amplified cardiac stem cells are regulated by Sca-1 signaling for efficient cardiovascular regeneration. J Cell Sci 120:1791–1800
Takamiya M, Haider KH, Ashraf M (2011) Identification and characterization of a novel multipotent sub-population of Sca-1+ cardiac progenitor cells for myocardial regeneration. PLoS One 6:e25265
Martin BJ, McClanahan TB, Van Wylen DG, Gallagher KP (1997) Effects of ischemia, preconditioning, and adenosine deaminase inhibition on interstitial adenosine levels and infarct size. Basic Res Cardiol 92:240–251
Willems L, Reichelt ME, Molina JG, Sun CX, Chunn JL, Ashton KJ, Schnermann J, Blackburn MR, Headrick JP (2006) Effects of adenosine deaminase and A1 receptor deficiency in normoxic and ischaemic mouse hearts. Cardiovasc Res 71:79–87
Fredholm BB (2007) Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ 14:1315–1323
Gharibi B, Abraham AA, Ham J, Evans BA (2012) Contrasting effects of A1 and A2b adenosine receptors on adipogenesis. Int J Obes (Lond) 36:397–406
Gharibi B, Abraham AA, Ham J, Evans BA (2011) Adenosine receptor subtype expression and activation influence the differentiation of mesenchymal stem cells to osteoblasts and adipocytes. J Bone Miner Res 26:2112–2124
Ham J, Evans BA (2012) An emerging role for adenosine and its receptors in bone homeostasis. Front Endocrinol (Lausanne) 3:113
Carroll SH, Wigner NA, Kulkarni N, Johnston-Cox H, Gerstenfeld LC, Ravid K (2012) A2B adenosine receptor promotes mesenchymal stem cell differentiation to osteoblasts and bone formation in vivo. J Biol Chem 287:15718–15727
Ciciarello M, Zini R, Rossi L, Salvestrini V, Ferrari D, Manfredini R, Lemoli RM (2013) Extracellular purines promote the differentiation of human bone marrow-derived mesenchymal stem cells to the osteogenic and adipogenic lineages. Stem Cells Dev 22:1097–1111
He W, Mazumder A, Wilder T, Cronstein BN (2013) Adenosine regulates bone metabolism via A1, A2A, and A2B receptors in bone marrow cells from normal humans and patients with multiple myeloma. FASEB J 27:3446–3454
Carlson S, Trial J, Soeller C, Entman ML (2011) Cardiac mesenchymal stem cells contribute to scar formation after myocardial infarction. Cardiovasc Res 91:99–107
Cieslik KA, Trial J, Entman ML (2011) Defective myofibroblast formation from mesenchymal stem cells in the aging murine heart rescue by activation of the AMPK pathway. Am J Pathol 179:1792–1806
Cieslik KA, Trial J, Carlson S, Taffet GE, Entman ML (2013) Aberrant differentiation of fibroblast progenitors contributes to fibrosis in the aged murine heart: role of elevated circulating insulin levels. FASEB J 27:1761–1771
van den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J (2010) Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol 7:30–37
Stefanovic B, Schnabl B, Brenner DA (2002) Inhibition of collagen alpha 1(I) expression by the 5’ stem-loop as a molecular decoy. J Biol Chem 277:18229–18237
Dubey RK, Gillespie DG, Mi Z, Jackson EK (1997) Exogenous and endogenous adenosine inhibits fetal calf serum-induced growth of rat cardiac fibroblasts: role of A2B receptors. Circulation 96:2656–2666
Dubey RK, Gillespie DG, Jackson EK (1998) Adenosine inhibits collagen and protein synthesis in cardiac fibroblasts: role of A2B receptors. Hypertension 31:943–948
Chen Y, Epperson S, Makhsudova L, Ito B, Suarez J, Dillmann W, Villarreal F (2004) Functional effects of enhancing or silencing adenosine A2b receptors in cardiac fibroblasts. Am J Physiol 287:H2478–H2486
Epperson SA, Brunton LL, Ramirez-Sanchez I, Villarreal F (2009) Adenosine receptors and second messenger signaling pathways in rat cardiac fibroblasts. Am J Physiol Cell Physiol 296:C1171–C1177
Villarreal F, Epperson SA, Ramirez-Sanchez I, Yamazaki KG, Brunton LL (2009) Regulation of cardiac fibroblast collagen synthesis by adenosine: roles for Epac and PI3K. Am J Physiol 296:C1178–C1184
Zhong H, Belardinelli L, Zeng D (2011) Pro-fibrotic role of the A2B adenosine receptor in human cardiac fibroblasts. J Card Fail 17:S65
Desmouliere A, Redard M, Darby I, Gabbiani G (1995) Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 146:56–66
Hecker L, Jagirdar R, Jin T, Thannickal VJ (2011) Reversible differentiation of myofibroblasts by MyoD. Exp Cell Res 317:1914–1921
Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, Iwaisako K, Moore-Morris T, Scott B, Tsukamoto H, Evans SM, Dillmann W, Glass CK, Brenner DA (2012) Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A 109:9448–9453
Garrison G, Huang SK, Okunishi K, Scott JP, Kumar Penke LR, Scruggs AM, Peters-Golden M (2013) Reversal of myofibroblast differentiation by prostaglandin E2. Am J Respir Cell Mol Biol 48:550–558
Wakeno M, Minamino T, Seguchi O, Okazaki H, Tsukamoto O, Okada K, Hirata A, Fujita M, Asanuma H, Kim J, Komamura K, Takashima S, Mochizuki N, Kitakaze M (2006) Long-term stimulation of adenosine A2b receptors begun after myocardial infarction prevents cardiac remodeling in rats. Circulation 114:1923–1932
Toldo S, Zhong H, Mezzaroma E, Van TB, Kannan H, Zeng D, Belardinelli L, Voelkel N, Abbate A (2012) GS-6201, a selective blocker of the A2B adenosine receptor, attenuates cardiac remodeling following acute myocardial infarction in the mouse. J Pharmacol Exp Ther 343:587–595
Maas JE, Koupenova M, Ravid K, Auchampach JA (2008) The A2B adenosine receptor contributes to post-infarction heart failure. Circulation 118:S946
Zhang H, Zhong H, Everett TH, Wilson E, Chang R, Zeng D, Belardinelli L, Olgin JE (2014) Blockade of A2B adenosine receptor reduces left ventricular dysfunction and ventricular arrhythmias 1 week after myocardial infarction in the rat model. Heart Rhythm 11:101–109
Novitskiy SV, Ryzhov S, Zaynagetdinov R, Goldstein AE, Huang Y, Tikhomirov OY, Blackburn MR, Biaggioni I, Carbone DP, Feoktistov I, Dikov MM (2008) Adenosine receptors in regulation of dendritic cell differentiation and function. Blood 112:1822–1831
Ryzhov S, Zaynagetdinov R, Goldstein AE, Novitskiy SV, Blackburn MR, Biaggioni I, Feoktistov I (2008) Effect of A2B adenosine receptor gene ablation on adenosine-dependent regulation of proinflammatory cytokines. J Pharmacol Exp Ther 324:694–700
Acknowledgements
This work was supported by the National Institutes of Health National Heart, Lung and Blood Institute [grant R01HL095787 and K08HL094703], National Cancer Institute [grant R01CA138923], American Heart Association Research Grant-in-Aid [13GRNT16580020], and Vanderbilt Clinical and Translational Science Award (CSTA) [grant UL1 RR024975-01] from the National Institutes of Health National Center for Research Resources (Vanderbilt Institute for Clinical and Translational Research CTSA grant VR5622).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 924 kb)
Rights and permissions
About this article
Cite this article
Ryzhov, S., Sung, B.H., Zhang, Q. et al. Role of adenosine A2B receptor signaling in contribution of cardiac mesenchymal stem-like cells to myocardial scar formation. Purinergic Signalling 10, 477–486 (2014). https://doi.org/10.1007/s11302-014-9410-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-014-9410-y