Abstract
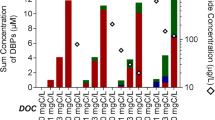
During chlorination and ozonation, a key concern is the conversion of bromide ions (Br−) to disinfection by-products (DBPs) such as trihalomethanes (THMs), haloacetic acids (HAAs), and bromate (BrO3 −). The objective of this study was to investigate the effects of water quality parameters (dissolved organic carbon (DOC), Br−, pH, and temperature) on the percent conversion of Br− to the known DBPs. Ten to twelve surface and ground waters having different DOC (1.2–10.6 mg/L) and Br− (7–312 μg/L) were subjected to bench scale chlorination and ozonation experiments at various pH (6.5–8.5) and temperature (15,∘C–25,∘C) conditions. The results show that the average percent of bromide incorporation was 24.5% for THMs and 9.5% for HAAs, and 12% for BrO3 − for the waters, respectively. In addition, the percent of bromide incorporation depends on source water pH. The percent of bromide incorporation increased with increasing pH (6.5 to 8.5) for THMs (18% to 28%) and BrO3 − (6.9% to 19%) and was insignificantly influenced by pH for HAAs. Coagulation using alum was effective in removing DBP reactive precursors such as DOC, which resulted in the smaller percentage of bromide incorporation for coagulated waters. The calculated average health risk factor for bromate was 4.3× 10−4 for the ten source waters, which is approximately 10 times higher than that for the average THMs (4.4× 10−5), respectively.
Similar content being viewed by others
References
Amy, G., Siddiqui, M., Ozekin, K., Zhu, H. and Wang, C.: 1998, ‘Empirically based models for predicting chlorination and ozonation by-products: Haloacetic acids, chloral hydrate, and bromate’, EPA Report CX 819579, USA.
Amy, G., Douville, C., Daw, B., Sohn, J., Galey, C., Gatel, D. and Cavard, J.: 2000, ‘Bromate formation under ozonation conditions to inactivate Cryptosporidium’, Water Science and Technology 41, 61–66.
Amy, G. L., Tan, L. and Davis, M. K.: 1991, ‘The effects of ozonation and activated carbon adsorption on trihalomethane speciation’, Water Research 25, 191–202.
Arora, H., LeChevallier, M. W. and Dixon, K. L.: 1997, ‘DBP occurrence survey’, Journal American Water Works Association 89, 60–68.
Bull, R. and Kopfler, F.: 1991, ‘Health Effect of Disinfectants and Disinfection By-Products’, American Water Works Association Research Foundation (AwwaRF), USA.
Clark, R. M., Thurnau, R. C., Sivaganesan, M. and Ringhand, P.: 2001, ‘Predicting the formation of chlorinated and brominated by-products’, Journal of Environmental Engineering-ASCE. 127, 493–501.
Echigo, S., Itoh, S., Natsui, T., Araki, T. and Ando, R.: 2004, ‘Contribution of brominated organic disinfection by-products to the mutagenicity of drinking water’, Water Science and Technology 50, 321–328.
Gibbons, J. and Laha, S.: 1999, ‘Water purification systems: A comparative analysis based on the occurrence of disinfection by-products’, Environmental Pollution. 106, 425–428.
von Gunten, U.: 2003, ‘Ozonation of drinking water: Part II. Disinfection and by-product formation in presence of bromide, iodine or chloine’, Water Research 37, 1469–1487.
Haas, C. N., Jacangelo, J. G., Bishop, M. M., Cameron, C. D., Chowdhury, Z. K. and Connell, G. F.: 1992, ‘Survey of water utility disinfection practices’, Journal American Water Works Association 84, 121–128.
Krasner, S. W., Glaze, W. H., Weinberg, H. S., Daniel, P. A. and Najm, I. N.: 1993, ‘Formation and control of bromate during ozonation of waters containing bromide’, Journal American Water Works Association 85, 73–81.
Lekkas, T. D. and Nikolaou, A. D.: 2004, ‘Development of predictive models for the formation of trihalomethanes and haloacetic acids during chlorination of bromide-rich water’, Water Quality Research Journal of Canada 39, 149–159.
Najm, I. N. and Krasner, S. W.: 1995, ‘Effects of bromide and nom on by-product formation’, Journal American Water Works Association 87, 106–115.
Owen, D. M., Amy, G. L., Chowdhury, Z. K., Paode, R., McCoy, G. and Viscosil, K.: 1995, ‘NOM - characterization and treatability’, Journal American Water Works Association 87, 46–63.
Pourmoghaddas, H., Stevens, A. A., Kinman, R. N., Dressman, R. C., Moore, L. A. and Ireland, J. C.: 1993, ‘Effect of bromide ion on formation of HAAs during chlorination’, Journal American Water Works Association 85, 82–87.
Rathbun, R. E.: 1996, ‘Bromine incorporation factors for trihalomethane formation for the Mississippi, Missouri, and Ohio Rivers’, Science of the Total Environment 192, 111–118.
Reckhow, D. A. and Singer, P. C.: 1990, ‘Chlorination by-products in drinking waters - from formation potentials to finished water concentrations’, Journal of American Water Works Association 82, 173–180.
Richardson, S. D., Thruston, A. D., Rav-Acha, C., Groisman, L., Popilevsky, I. and Juraev, O.: 2003, ‘Tribromopyrrole, brominated acids, and other disinfection byproducts produced by disinfection of drinking water rich in bromide’, Environmental Science & Technology 37, 3782–3793.
Rook, J. J.: 1977, ‘Chlorination reactions of fulvic acids in natural waters’, Environmental Science & Technolog 11, 478–482.
Siddiqui, M.: 1992, ‘Ozone-Bromide Interactions in Water Treatment’, Ph.D. Dissertation. University of Arizona.
Siddiqui, M. S. and Amy, G. L.: 1993, ‘Factors affecting DBP formation during ozone bromide reactions’, Journal American Water Works Association 85, 63–72.
Sinha, S., Yoon, Y., Amy, G. and Yoon, J.: 2004, ‘Determining the effectiveness of conventional and alternative coagulants through effective characterization schemes’, Chemosphere 57, 1115–1122.
Sohn, J., Sinha, S., Zhu, H. and Amy, G.: 1997, ‘The role of coagulation in DBP-related risk reduction’, American Water Works Association Annual Conference. Denver, USA.
Song, R., Westerhoff, P., Minear, R. and Amy, G.: 1997, ‘Bromate minimization during ozonation’, Journal American Water Works Association 89, 69–78.
USEPA: 1994, ‘Drinking water; national primary drinking water regulations: disinfectants and disinfection byproducts’, Proposed Rule, Fed. Reg. 59(145), 38668.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sohn, J., Amy, G. & Yoon, Y. Bromide Ion Incorporation Into Brominated Disinfection By-Products. Water Air Soil Pollut 174, 265–277 (2006). https://doi.org/10.1007/s11270-006-9104-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-006-9104-3