Abstract
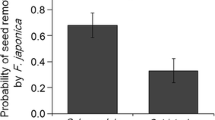
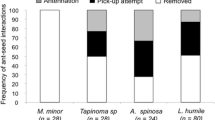
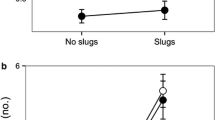
This study assesses the dispersal mechanisms of the narrow endemic Polygala vayredae, analysing the functioning of its dispersal syndromes (anemochory and myrmecochory), the spatio-temporal variability of the disperser assemblage, foraging behaviour and dispersal ability, and the role of the elaiosome in ant attraction and seed germination. The dispersion of diaspores begins when either (1) capsules or seeds fall beneath the mother plant (barochory) or (2) the seeds are directly collected in the suspended capsules by ants (myrmecochory). As capsules frequently open and expose/disseminate seeds before leaving the mother plant, the adaptation for anemochory appears to be reduced and rarely functional, possibly with only occasional events of long-distance dispersal (e.g. under extreme weather conditions). P. vayredae is essentially myrmecochorous and a diverse array of ant species are involved in seed manipulation, with the elaiosome playing a major role in ant attraction. From the plant’s perspective for dispersal, the majority of ant species had a positive interaction with the seeds, but negative and potential neutral interactions were also observed. Overall, dispersal distances were limited and were mainly determined by ant body size. The frequency of interactions and the ant assemblage varied significantly both spatially and temporally, and these factors may have an effect on directing or disrupting the selection of plant traits. Low seed predation and similar germination rates of intact seeds compared with seeds without elaiosome indicate that seed predator avoidance and seed germination improvement after ant manipulation are not among the selective advantages of myrmecochory operating at present. Dispersal mechanisms that enhance seed dispersal within the population and only occasionally lead to long-distance dispersal events, along with the rarity and patchiness of suitable habitats, may be the main factors explaining the actual density and narrow distribution of this species.



Similar content being viewed by others
References
Alcántara JM, Rey PJ, Manzaneda AJ, Boulay R, Ramírez JM, Fedriani JM (2007) Geographic variation in the adaptive landscape for seed size at dispersal in the myrmecochorous Helleborus foetidus. Evol Ecol 21:411–430
Andersen AN (1992) Regulation of ‘momentary’ diversity by dominant species in exceptionally rich ant communities of the Australian seasonal tropics. Am Nat 140:401–420
Andersen AN, Majer JD (2004) Ants show the way down under: invertebrates as bioindicators in land management. Front Ecol Environ 2:291–298
Beattie A (1985) The evolutionary ecology of ant-plant mutualisms. Cambridge University Press, Cambridge
Berg RY (1975) Myrmecochorous plants in Australia and their dispersal by ants. Aust J Bot 23:475–508
Bolós A (1946) La Polygala vayredae Costa, endemismo pirenaico. Collect Bot 1:7–93
Boulay R, Coll-Toledano J, Cerdá X (2006) Geographic variations in Helleborus foetidus elaiosome lipid composition: implications for dispersal by ants. Chemoecology 16:1–7
Boulay R, Carro F, Soriguer RC, Cerdá X (2007) Synchrony between fruit maturation and effective dispersers’ foraging activity increases seed protection against seed predators. Proc R Soc Biol Sci Ser B 274:2515–2522
Boyd RS (2001) Ecological benefits of myrmecochory for the endangered chaparral shrub Fremontodendron decumbens (Sterculiaceae). Am J Bot 88:234–241
Castro S (2007) Reproductive biology and conservation of the endemic Polygala vayredae. PhD Dissertation, University of Aveiro
Castro S, Silveira P, Navarro L (2008) How flower biology and breeding system affect the reproductive success of the narrow endemic Polygala vayredae Costa (Polygalaceae). Bot J Linn Soc 157:67–81
Castro S, Silva S, Stanescu I, Silveira P, Navarro L, Santos C (2009a) Pistil anatomy and pollen tube development in Polygala vayredae Costa (Polygalaceae). Plant Biol 11:405–416
Castro S, Silveira P, Navarro L (2009b) Floral traits variation, legitimate pollination, and nectar robbing in Polygala vayredae (Polygalaceae). Ecol Res 24:47–55
Cerdá X, Retana J, Manzaneda A (1998) The role of competition by dominants and temperature in the foraging of subordinate species in Mediterranean ant communities. Oecologia 117:404–412
Culver DC, Beattie AJ (1978) Myrmecochory in Viola: dynamics of seed-ant interactions in some West Virginia species. J Ecol 66:53–72
Culver DC, Beattie AJ (1980) The fate of Viola seeds dispersed by ants. Am J Bot 67:710–714
Edwards W, Dunlop M, Rodgerson L (2006) The evolution of rewards: seed dispersal, seed size and elaiosome size. J Ecol 94:687–694
Feener DH, Schupp EW (1998) Effect of treefall gaps on the patchiness and species richness of Neotropical ant assemblages. Oecologia 116:191–201
Forest F, Chase MW, Persson C, Crane PR, Hawkins JA (2007) The role of biotic and abiotic factors in evolution of ant dispersal in the milkwort family (Polygalaceae). Evolution 61:1675–1694
Gammans N, Bullock JM, Schönrogge K (2005) Ant benefits in a seed dispersal mutualism. Oecologia 146:43–49
Garrido JL, Rey PJ, Cerdá X, Herrera CM (2002) Geographical variation in diaspore traits of an ant-dispersed plant (Helleborus foetidus): are ant community composition and diaspore traits correlated? J Ecol 90:446–455
Giladi I (2006) Choosing benefits or partners: a review of the evidence for the evolution of myrmecochory. Oikos 112:481–492
Gómez C, Espadaler X (1998a) Seed dispersal curve of a Mediterranean myrmecochore: influence of ant size and the distance to nests. Ecol Res 13:347–354
Gómez C, Espadaler X (1998b) Myrmecochorous dispersal distances: a world survey. J Biogeogr 25:573–580
Gómez C, Pons P, Bas JM (2003) Effects of the argentine ant Linepithema humile on seed dispersal and seedling emergence of Rhamnus alaternus. Ecography 26:532–538
Gorb E, Gorb S (2003) Seed dispersal by ants in a deciduous forest ecosystem. Kluwer Academic Publishers, Dordrecht, Boston, London
Guitián J, Garrido JL (2006) Is early flowering in myrmecochorous plants an adaptation for ant dispersal? Plant Species Biol 21:165–171
Guitián P, Medrano M, Guitián J (2003) Seed dispersal in Erythronium dens-canis L. (Liliaceae): variation among habitats in a myrmecochorous plant. Plant Ecol 169:171–177
Handel SN (1978) Competitive relationship of three woodland sedges and its bearing on evolution of ant-dispersal of Carex pedunculata. Evolution 32:151–163
Heithaus ER (1981) Seed predation by rodents on three ant-dispersed plants. Ecology 62:136–145
Higashi S, Tsuyuzaki S, Ohara M, Ito F (1989) Adaptive advantages of ant-dispersed seeds in the myrmecochorous plant Trillium tschonoskii (Liliaceae). Oikos 54:389–394
Higgins SI, Nathan R, Cain ML (2003) Are long-distance dispersal events in plants usually caused by nonstandard means of dispersal? Ecology 84:1945–1956
Horvitz CC, Beattie AJ (1980) Ant dispersal of Calathea (Marantaceae) seeds by carnivorous ponerines (Formicidae) in a tropical rain forest. Am J Bot 67:321–326
Horvitz CC, Schemske DW (1986) Ant-nest soil and seedling growth in a neotropical ant-dispersed herb. Oecologia 70:318–320
Hughes L, Westoby M (1992a) Fate of seeds adapted for dispersal by ants in Australian sclerophyll vegetation. Ecology 73:1285–1299
Hughes L, Westoby M (1992b) Effect of diaspore characteristics on removal of seeds adapted for dispersal by ants. Ecology 73:1300–1312
Hughes L, Westoby M, Jurado E (1994) Convergence of elaiosomes and insect prey: evidence from ant foraging behavior and fatty acid composition. Funct Ecol 8:358–365
Imbert E (2006) Dispersal by ants in Centaurea corymbosa (Asteraceae): what is the elaiosome for? Plant Species Biol 21:109–117
Lack AJ, Kay QON (1987) Genetic structure, gene flow and reproductive ecology in sand-dune populations of Polygala vulgaris. J Ecol 75:259–276
Lanza J, Schmitt MA, Awad AB (1992) Comparative chemistry of elaiosomes of 3 species of Trillium. J Chem Ecol 18:209–221
Lassau SA, Hochuli DF (2004) Effects of habitat complexity on ant assemblages. Ecography 27:157–164
Lesica P, Yurkewycz R, Crone EE (2006) Rare plants are common where you find them. Am J Bot 93:454–459
Manzaneda AJ, Rey PJ (2009) Assessing ecological specialization of an ant-seed dispersal mutualism through a wide geographic range. Ecology
Manzaneda AJ, Fedriani JM, Rey PJ (2005) Adaptive advantages of myrmecochory: the predator-avoidance hypothesis tested over a wide geographic range. Ecography 28:583–592
Manzaneda AJ, Rey PJ, Boulay R (2007) Geographic and temporal variation in the ant-seed dispersal assemblage of the perennial herb Helleborus foetidus L. (Ranunculaceae). Biol J Linn Soc 92:135–150
Mark S, Olesen JM (1996) Importance of elaiosome size to removal of ant-dispersed seeds. Oecologia 107:95–101
Morales MA, Heithaus ER (1998) Food from seed dispersal mutualism shifts sex ratios in colonies of the ant Aphaenogaster rudis. Ecology 79:734–739
Münzbergova Z (2004) Effect of spatial scale on factors limiting species distributions in dry grassland fragments. J Ecol 92:854–867
Nathan R, Sapir N, Trakhtenbrot A, Katul GG, Bohrer G, Otte M, Avissar R, Soons MB, Horn HS, Wikelski M, Levin SA (2005) Long-distance biological transport processes through the air: can nature’s complexity be unfolded in silico? Divers Distrib 11:131–137
Navarro L, Guitián J (2003) Seed germination and seedling survival of two threatened endemic species of the northwest Iberian Peninsula. Biol Conserv 109:313–320
Ness JH, Morin DF (2008) Forest edges and landscape history shape interactions between plants, seed-dispersing ants and seed predators. Biol Conserv 141:838–847
Ness JH, Bronstein JL, Andersen AN, Holland JN (2004) Ant body size predicts dispersal distance of ant-adapted seeds: implications of small-ant invasions. Ecology 85:1244–1250
Oberrath R, Bohning-Gaese K (2002) Phenological adaptations of ant-dispersed plants to seasonal variation in ant activity. Ecology 83:1412–1420
Oostermeijer JGB (1989) Myrmecochory in Polygala vulgaris L., Luzula campestris (L.) DC. and Viola curtisii Forster in a Dutch dune area. Oecologia 78:302–311
Retana J, Cerdá X (2000) Patterns of diversity and composition of Mediterranean ground ant communities: tracking spatial and temporal variability in the thermal environment. Oecologia 123:436–444
Rey PJ, Manzaneda AJ (2007) Geographical variation in the determinants of seed dispersal success of a myrmecochorous herb. J Ecol 95:1381–1393
Rey PJ, Ramírez JM, Sánchez-Lafuente AM (2006) Seed- vs. micro-site limited recruitment in a myrmecochorous herb. Plant Ecol 184:213–222
Rico-Gray V, García-Franco JG, Palacios-Rios M, Díaz-Castelazo C, Parra-Tabla V, Navarro JA (1998) Geographical and seasonal variation in the richness of ant-plant interactions in México. Biotropica 30:190–200
Sernander R (1906) Entwurf einer Monographie der Europaischen Myrmekochoren. K Sv Vetensk Akad Handl 41:1–140
Thompson JN (1994) The geographic mosaic of evolving interactions. In: Leather SR, Watt AD, Mills NJ, Walters KFA (eds) Individuals, populations and patterns in ecology. Intercept Press, Andover
Thompson JN (2005) The geographic mosaic of coevolution. University of Chicago Press, Chicago
van der Pijl L (1982) Principles of dispersal in higher plants. Springer-Verlag, Berlin, Heidelberg, New York
Van Der Veken S, Rogister J, Verheyen K, Hermy M, Nathan RAN (2007) Over the (range) edge: a 45-year transplant experiment with the perennial forest herb Hyacinthoides non-scripta. J Ecol 95:343–351
van Roosmalen MGM (1985) Fruits of the Guianan flora. Utrecht University, Utecht
Acknowledgements
The authors thank the Departament de Medi Ambient of Generalitat de Catalunya and the Consorci d’Alta Garrotxa for allowing this research and Parc Natural de la Zona Volcànica de la Garrotxa for all the assistance, namely Mònica Canal for field support. Kiko Gómez is also thanked for the photographs of ant specimens. The Portuguese Foundation for Science and Technology financed the work of Sílvia Castro (BD/10901/2002 and BPD/41200/2007) and João Loureiro (BPD/36601/2007) and the Spanish Ministerio de Educación y Ciencia financed the work of Victoria Ferrero (AP-2004-6394). This study was also partially financed under Grants PGIDT04PXIC31003PN from the Xunta de Galicia, CGL2006-13847-CO2-02 from the Spanish Dirección General de Investigación, Ciencia y Técnología and FEDER funds from the European Union to Luis Navarro.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castro, S., Ferrero, V., Loureiro, J. et al. Dispersal mechanisms of the narrow endemic Polygala vayredae: dispersal syndromes and spatio-temporal variations in ant dispersal assemblages. Plant Ecol 207, 359–372 (2010). https://doi.org/10.1007/s11258-009-9679-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-009-9679-z