Abstract
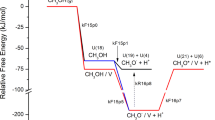
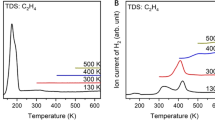
The temperature programmed desorption of acetaldehyde adsorbed on partially reduced CeO2(111) has been studied in detail using microkinetic modeling based on self-consistent, periodic density functional theory calculations at the GGA-PW91+U level. Previous experimental studies (Chen et al. J. Phys. Chem. C 115: 3385, 2011; Calaza et al. J. Am. Chem. Soc. 134: 18034, 2012) have shown that, whereas on fully oxidized CeO2(111) acetaldehyde desorbs molecularly with a peak temperature of 210 K, the polymerization and enolization of acetaldehyde dominate the surface reactivity on partially reduced CeO2(111), resulting in acetaldehyde desorption at higher temperatures. Here we propose a comprehensive reaction mechanism that is consistent with the spectroscopic evidence of the identities of the surface intermediates and with the observed desorption activities, including the formation of ethylene and acetylene. Besides acetaldehyde (CH3CHO) and its enolate (CH2CHO), several other C2H x O species are proposed as key intermediates which are not seen spectroscopically, including ethoxy (CH3CH2O), ethyleneoxy (CH2CH2O), and formylmethylene (CHCHO). Our study suggests that oxygen vacancies play the critical role of activating the carbonyl bond and thereby facilitating β C–H bond scissions in acetaldehyde, leading to enolization, intermolecular hydrogen transfer, deoxygenation, and potentially C–C coupling reactions.






Similar content being viewed by others
Change history
27 February 2019
The original version of this article unfortunately contained an error in Fig.��6. The authors would like to correct the error with this erratum. The errors were due to the missing of a factor of 2 in the rate expression for the surface coverage of atomic H when it desorbs as H2, and to incorrect settings for some of the initial coverages, in the microkinetic model. A corrected version is shown here as Fig.��6, with the same caption as before.
References
Kašpar J, Fornasiero P, Graziani M (1999) Use of CeO2-based oxides in the three-way catalysis. Catal Today 50:285–298
Bunluesin T, Gorte RJ, Graham GW (1998) Studies of the water-gas-shift reaction on ceria-supported Pt, Pd, and Rh: implications for oxygen-storage properties. Appl Catal B-Environ 15:107–114
Rodriguez JA, Ma S, Liu P, Hrbek J, Evans J, Pérez M (2007) Activity of CeOx and TiOx nanoparticles grown on Au(111) in the water-gas shift reaction. Science 318:1757–1760
Deluga GA, Salge JR, Schmidt LD, Verykios XE (2004) Renewable hydrogen from ethanol by autothermal reforming. Science 303:993–997
Wang SB, Lu GQ (1998) Role of CeO2 in Ni/CeO2–Al2O3 catalysts for carbon dioxide reforming of methane. Appl Catal B-Environ 19:267–277
Graciani J, Mudiyanselage K, Xu F, Baber AE, Evans J, Senanayake SD, Stacchiola DJ, Liu P, Hrbek J, Sanz JF, Rodriguez JA (2014) Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2. Science 345:546–550
Aneggi E, de Leitenburg C, Dolcetti G, Trovarelli A (2006) Promotional effect of rare earths and transition metals in the combustion of diesel soot over CeO2 and CeO2–ZrO2. Catal Today 114:40–47
Gutiérrez-Ortiz JI, de Rivas B, López-Fonseca R, González-Velasco JR (2006) Catalytic purification of waste gases containing VOC mixtures with Ce/Zr solid solutions. Appl Catal B-Environ 65:191–200
Trovarelli A, Fornasiero P (2013) Catalysis by ceria and related materials, vol 12, 2nd edn. Imperial College Press, London
Resasco DE (2011) What should we demand from the catalysts responsible for upgrading biomass pyrolysis oil? J Phys Chem Lett 2:2294–2295
Gürbüz EI, Kunkes EL, Dumesic JA (2010) Integration of C–C coupling reactions of biomass-derived oxygenates to fuel-grade compounds. Appl Catal B-Environ 94:134–141
Mohan D, Pittman CU, Steele PH (2006) Pyrolysis of wood/biomass for bio-oil: a critical review. Energ Fuel 20:848–889
Huber GW, Iborra S, Corma A (2006) Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering. Chem Rev 106:4044–4098
Alonso DM, Bond JQ, Dumesic JA (2010) Catalytic conversion of biomass to biofuels. Green Chem 12:1493–1513
Pham TN, Sooknoi T, Crossley SP, Resasco DE (2013) Ketonization of carboxylic acids: mechanisms, catalysts, and implications for biomass conversion. ACS Catal 3:2456–2473
Pestman R, Koster RM, van Duijne A, Pieterse JAZ, Ponec V (1997) Reactions of carboxylic acids on oxides: 2. bimolecular reaction of aliphatic acids to ketones. J Catal 168:265–272
Kunkes EL, Simonetti DA, West RM, Serrano-Ruiz JC, Gärtner CA, Dumesic JA (2008) Catalytic conversion of biomass to monofunctional hydrocarbons and targeted liquid-fuel classes. Science 322:417–421
Idriss H, Diagne C, Hindermann JP, Kiennemann A, Barteau MA (1995) Reactions of acetaldehyde on CeO2 and CeO2-supported catalysts. J Catal 155:219–237
Raskó J, Kiss J (2005) Adsorption and surface reactions of acetaldehyde on TiO2, CeO2 and Al2O3. Appl Catal A-Gen 287:252–260
Chen TL, Mullins DR (2011) Adsorption and reaction of acetaldehyde over CeOx(111) thin films. J Phys Chem C 115:3385–3392
Calaza FC, Xu Y, Mullins DR, Overbury SH (2012) Oxygen vacancy-assisted coupling and enolization of acetaldehyde on CeO2(111). J Am Chem Soc 134:18034–18045
Mann AKP, Wu ZL, Calaza FC, Overbury SH (2014) Adsorption and reaction of acetaldehyde on shape-controlled CeO2 nanocrystals: elucidation of structure–function relationships. ACS Catal 4:2437–2448
Kresse G, Furthmüller J (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B 54:11169
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1992) Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys Rev B 46:6671
Kresse G, Joubert D (1999) From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B 59:1758
Monkhorst HJ, Pack JD (1976) Special points for Brillouin-zone integrations. Phys Rev B 13:5188
Neugebauer J, Scheffler M (1992) Adsorbate-substrate and adsorbate-adsorbate interactions of Na and K adlayers on Al(111). Phys Rev B 46:16067
Henkelman G, Uberuaga BP, Jónsson H (2000) A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J Chem Phys 113:9901
Smidstrup S, Pedersen A, Stokbro K, Jónsson H (2014) Improved initial guess for minimum energy path calculations. J Chem Phys 140:214106
Henkelman G, Jónsson H (1999) A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives. J Chem Phys 111:7010
Heyden A, Bell AT, Keil FJ (2005) Efficient methods for finding transition states in chemical reactions: comparison of improved dimer method and partitioned rational function optimization method. J Chem Phys 123:224101
Dudarev SL, Botton GA, Savrasov SY, Humphreys CJ, Sutton AP (1998) Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA + U study. Phys Rev B 57:1505
Kresse G, Blaha P, Da Silva JLF, Ganduglia-Pirovano MV (2005) Comment on “Taming multiple valency with density functionals: a case study of defective ceria”. Phys Rev B 72:237101
Gordon WO, Xu Y, Mullins DR, Overbury SH (2009) Temperature evolution of structure and bonding of formic acid and formate on fully oxidized and highly reduced CeO2(111). Phys Chem Chem Phys 11:11171–11183
Redhead PA (1962) Thermal desorption of gases. Vacuum 12:203–211
Loschen C, Carrasco J, Neyman KM, Illas F (2007) First-principles LDA + U and GGA + U study of cerium oxides: dependence on the effective U parameter. Phys Rev B 75:035115
Lutfalla S, Shapovalov V, Bell AT (2011) Calibration of the DFT/GGA + U method for determination of reduction energies for transition and rare earth metal oxides of Ti, V, Mo, and Ce. J Chem Theory Comput 7:2218–2223
Fabris S, Vicario G, Balducci G, de Gironcoli S, Baroni S (2005) Electronic and atomistic structures of clean and reduced ceria surfaces. J Phys Chem B 109:22860–22867
Lu DY, Liu P (2014) Rationalization of the Hubbard U parameter in CeOx from first principles: unveiling the role of local structure in screening. J Chem Phys 140:084101
Paier J, Penschke C, Sauer J (2013) Oxygen defects and surface chemistry of ceria: quantum chemical studies compared to experiment. Chem Rev 113:3949–3985
Zhou J, Baddorf AP, Mullins DR, Overbury SH (2008) Growth and characterization of Rh and Pd nanoparticles on oxidized and reduced CeOx(111) thin films by scanning tunneling microscopy. J Phys Chem C 112:9336–9345
Esch F, Fabris S, Zhou L, Montini T, Africh C, Fornasiero P, Comelli G, Rosei R (2005) Electron localization determines defect formation on ceria substrates. Science 309:752–755
Kullgren J, Wolf MJ, Castleton CWM, Mitev P, Briels WJ, Hermansson K (2014) Oxygen vacancies versus fluorine at CeO2(111): a case of mistaken identity? Phys Rev Lett 112:156102
Steinfeld JI, Francisco JS, Hase WL (1999) Chemical kinetics and dynamics. Prentice Hall, Englewood Cliffs
Andersson MP, Abild-Pedersen E, Remediakis IN, Bligaard T, Jones G, Engbæk J, Lytken O, Horch S, Nielsen JH, Sehested J, Rostrup-Nielsen JR, Nørskov JK, Chorkendorff I (2008) Structure sensitivity of the methanation reaction: H2-induced CO dissociation on nickel surfaces. J Catal 255:6–19
Olcay H, Xu Y, Huber GW (2014) Effects of hydrogen and water on the activity and selectivity of acetic acid hydrogenation on ruthenium. Green Chem 16:911–924
Mizukami T (1965) Physico-chemical studies on acetaldehyde polymerization at high pressure and low temperature: 2. kinetics of polymerization of acetaldehyde. Rev Phys Chem Jpn 35:60
Davis JL, Barteau MA (1989) Polymerization and decarbonylation reactions of aldehydes on the Pd(111) surface. J Am Chem Soc 111:1782–1792
Momma K, Izumi F (2008) VESTA: a three-dimensional visualization system for electronic and structural analysis. J Appl Crystallogr 41:653–658
Carrasco J, Vilé G, Fernández-Torre D, Pérez R, Pérez-Ramírez J, Ganduglia-Pirovano MV (2014) Molecular-level understanding of CeO2 as a catalyst for partial alkyne hydrogenation. J Phys Chem C 118:5352–5360
Vicario G, Balducci G, Fabris S, de Gironcoli S, Baroni S (2006) Interaction of hydrogen with cerium oxide surfaces: a quantum mechanical computational study. J Phys Chem B 110:19380–19385
Popa C, Ganduglia-Pirovano MV, Sauer J (2011) Periodic density functional theory study of VOn species supported on the CeO2(111) surface. J Phys Chem C 115:7399–7410
Fernández-Torre D, Carrasco J, Ganduglia-Pirovano MV, Pérez R (2014) Hydrogen activation, diffusion, and clustering on CeO2(111): a DFT + U study. J Chem Phys 141:014703
Wu XP, Gong XQ, Lu GZ (2015) Role of oxygen vacancies in the surface evolution of H at CeO2(111): a charge modification effect. Phys Chem Chem Phys 17:3544–3549
Klimeš J, Bowler DR, Michaelides A (2011) Van der Waals density functionals applied to solids. Phys Rev B 83:195131
Mullins DR, Albrecht PM, Calaza F (2013) Variations in reactivity on different crystallographic orientations of cerium oxide. Top Catal 56:1345–1362
Christiansen MA, Mpourmpakis G, Vlachos DG (2015) DFT-driven multi-site microkinetic modeling of ethanol conversion to ethylene and diethyl ether on γ-Al2O3(111). J Catal 323:121–131
Beste A, Overbury SH (2015) Pathways for ethanol dehydrogenation and dehydration catalyzed by ceria (111) and (100) surfaces. J Phys Chem C 119:2447–2455
Mullins DR, Senanayake SD, Chen TL (2010) Adsorption and reaction of C1–C3 alcohols over CeOx(111) thin films. J Phys Chem C 114:17112–17119
Chen BH, Ma YS, Ding LB, Xu LS, Wu ZF, Yuan Q, Huang WX (2013) Reactivity of hydroxyls and water on a CeO2(111) thin film surface: the role of oxygen vacancy. J Phys Chem C 117:5800–5810
Calaza FC, Chen TL, Mullins DR, Xu Y, Overbury SH (2015) Reactivity and reaction intermediates for acetic acid adsorbed on CeO2(111). Catal Today 253:65–76
García-Melchor M, López N (2014) Homolytic products from heterolytic paths in H2 dissociation on metal oxides: the example of CeO2. J Phys Chem C 118:10921–10926
Gilkey MJ, Xu BJ (2016) Heterogeneous catalytic transfer hydrogenation as an effective pathway in biomass upgrading. ACS Catal 6:1420–1436
Acknowledgments
This work was supported by the Louisiana Experimental Program to Stimulate Competitive Research (EPSCoR) Program, funded by the National Science Foundation and the Louisiana Board of Regents Support Fund, and has used high performance computational resources provided by Louisiana State University, the Center for Nanophase Materials Sciences at Oak Ridge National Laboratory, which is a U.S. Department of Energy (US-DOE), Office of Science User Facility, and the National Energy Research Scientific Computing Center, which is supported by the Office of Science of US-DOE under Contract No. DE-AC02-05CH11231.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, C., Xu, Y. Simulated Temperature Programmed Desorption of Acetaldehyde on CeO2(111): Evidence for the Role of Oxygen Vacancy and Hydrogen Transfer. Top Catal 60, 446–458 (2017). https://doi.org/10.1007/s11244-016-0703-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-016-0703-y