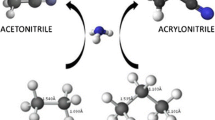
Abstract
The selective ammoxidation of propane into acrylonitrile catalyzed by the bulk Mo–V–Te–Nb–O system has received significant attention because it is more environmentally benign than the current process of propene ammoxidation and relies on more abundant propane feedstock. The reaction mechanism is proposed to consist of a series of elementary steps including propane oxidative dehydrogenation, ammonia and O2 activation, and NHx insertion into C3 intermediates. In this study density functional theory calculations have been performed to investigate the energetics of ammonia adsorption and activation in the proposed active center in the ab plane of the M1 phase. The formation of NH x (x = 0, 1, 2, 3) species is found to be highly favored on reduced, oxo-depleted metal sites. The reduced Mo site is determined to be the most favorable site for ammonia activation by comparing the reaction energy profiles for the sequential dehydrogenation of ammonia on the various metal sites. The activation barrier for the initial H abstraction from ammonia was found to depend strongly on the surface sites that stabilize H and NH2, and is as low as 0.28 eV when NH2 is stabilized by the reduced Mo site and H is abstracted by the telluryl oxo group. The subsequent step of surface NH insertion into a π-allyl gas intermediate was also found to have a low activation energy barrier of 0.03 eV on the reduced Mo site.




Similar content being viewed by others
References
Shiju NR, Guliants VV, Overbury SH, Rondinone AJ (2008) ChemSusChem 1:519–523
Tsuji H, Oshima K, Koyasu Y (2003) Chem Mater 15:2112–2114
Grasselli RK (2002) Top Catal 21:79–88
Hatano M, Kayo A (1991) Catalytic conversion of alkanes to nitriles, and a catalyst therefor, U.S Patent 5,049,692
Desanto P, Buttrey DJ, Grasselli RK, Lugmair CG, Volpe AF, Toby BH, Vogt T (2004) Z Krist 219:152–165
Korovchenko P, Shiju NR, Dozier AK, Graham UM, Guerrero-Pérez MO, Guliants VV (2008) Top Catal 50:43–51
Grasselli RK, Burrington JD, Buttrey DJ, Desanto P, Lugmair CG, Volpe AF, Weingand T (2003) Top Catal 23:5–22
Grasselli RK, Lugmair CG, Volpe AF (2011) Top Catal 54:595–604
Grasselli RK, Buttrey DJ, DeSanto P, Burrington JD, Lugmair CG, Volpe AF, Weingand T (2004) Catal Today 91–92:251–258
Chenoweth K, van Duin ACT, Goddard WA III (2009) Angew Chem Int Ed 48:7630–7634
Goddard WA III, Chenoweth K, Pudar S, van Duin ACT, Cheng M-J (2008) Top Catal 50:2–18
Goddard WA III, Liu L, Mueller JE, Pudar S, Nielsen RJ (2011) Top Catal 54:659–668
Goddard WA III, Mueller JE, Chenoweth K, van Duin ACT (2010) Catal Today 157:71–76
Fu G, Xu X, Sautet P (2012) Angew Chem Int Ed 51:12854–12858
Muthukumar K, Yu J, Xu Y, Guliants VV (2011) Top Catal 54:605–613
Govindasamy A, Muthukumar K, Yu J, Xu Y, Guliants VV (2010) J Phys Chem C 114:4544–4549
Yu J, Xu Y, Guliants VV (2014) Top Catal. doi:10.1016/j.cattod.2014.02.053
Watanabe N, Ueda W (2006) Ind Eng Chem Res 45:607–614
Grasselli RK (2005) Catal Today 99:23–31
Rozanska X, Fortrie R, Sauer J (2007) J Phys Chem C 111:6041–6050
Desanto P, Buttrey DJ, Grasselli RK, Lugmair CG, Volpe AF, Toby BH, Vogt T (2003) Top Catal 23:23–38
Yu J, Woo J, Borisevich A, Xu Y, Guliants VV (2012) Catal Commun 29:68–72
Perdew J, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865–3868
Kresse G, Furthmüller J (1996) Phys Rev B 54:11169–11186
Kresse G, Hafner J (1993) Phys Rev B 47:558–561
Kresse G, Hafner J (1994) Phys Rev B 49:14251–14268
Henkelman G, Arnaldsson A, Jónsson H (2006) Comput Mater Sci 36:354–360
Henkelman G, Jόnsson H (1999) J Chem Phys 111:7010–7021
Bader RFW (1990) Atoms in molecules—a quantum theory. Oxford University Press, Oxford
Rojas E, Calatayud M, Guerrero-Pérez MO, Bañares MA (2010) Catal Today 158:178–185
Yao H, Chen Y, Wei Y, Zhao Z, Liu Z, Xu C (2012) Surf Sci 606:1739–1748
Gruber M, Hermann K (2013) J Chem Phys 138:194701
Wachs IE, Jehng J-M, Ueda W (2005) J Phys Chem B 109:2275–2284
Sundararajan K, Sankaran K, Kavitha V (2008) J Mol Struct 876:240–249
Grasselli RK, Brazdil JF, Burrington JD (1984) Proc. 3rd Ind. Symp. Ind. Uses Selenium Tellurium. Stockholm, Sweden, p 183
Getsoian AB, Shapovalov V, Bell AT (2013) J Phys Chem C 17:7123–7137
Alexopoulos K, Reyniers M-F, Marin GB (2012) J Catal 295:195–206
Alexopoulos K, Reyniers M-F, Marin GB (2012) J Catal 289:127–139
Acknowledgments
This study was supported by the Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, U.S. Department of Energy, under Grant DE-FG02-04ER15604. Part of the DFT studies were performed at the Center for Nanophase Materials Sciences, which is sponsored at Oak Ridge National Laboratory (ORNL) by the Scientific User Facilities Division, Office of Basic Energy Sciences, U.S. Department of Energy. The resources of the National Energy Research Scientific Computing Center, supported by DOE Office of Science under Contract DE-AC02-05CH11231, are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, J., Xu, Y. & Guliants, V.V. Propane Ammoxidation over Mo–V–Te–Nb–O M1 Phase Investigated by DFT: Elementary Steps of Ammonia Adsorption, Activation and NH Insertion into π-Allyl Intermediate. Top Catal 57, 1145–1151 (2014). https://doi.org/10.1007/s11244-014-0280-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-014-0280-x