Abstract
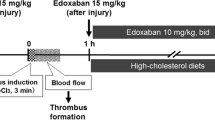
Heparin and low-molecular weight heparin (LMWH) are complex, heterogeneous polysaccharides used in the treatment of arterial and venous thrombosis. M118 is a novel LMWH with low polydispersity and pronounced anti-Xa and anti-thrombin (IIa) activity as compared to current LMWHs. To determine if M118 is effective in preventing thrombosis in the setting of a vascular plaque, apolipoprotein E knockout mice fed a high fat diet were injected with M118, enoxaparin, unfractionated heparin, or saline control and examined for arterial thrombosis using a rose bengal laser induced carotid artery injury model. M118 significantly increased the time to occlusion as compared to control and unfractionated heparin but not compared to enoxaparin although fewer M118 treated animals had any vascular occlusion present at the time of protocol completion. Platelet-neutrophil aggregates were studied by flow cytometry and were found to be decreased with M118 as compared to enoxaparin. This is the first published report examining M118, a novel LMWH designed to have low polydispersity and enhanced anticoagulant activity. In an animal model of vascular plaque, M118 is a potent inhibitor of arterial thrombosis and, despite lower in vivo anti-Xa and anti-IIa activity levels, M118 was superior to UFH in the prevention of arterial thrombosis.





Similar content being viewed by others
References
Freedman JE (2005) Molecular regulation of platelet-dependent thrombosis. Circulation 112:2725–2734. doi:10.1161/CIRCULATIONAHA.104.494468
Bates SM, Weitz JI (2005) New anticoagulants: beyond heparin, low-molecular-weight heparin and warfarin. Br J Pharmacol 144:1017–1028. doi:10.1038/sj.bjp.0706153
Bates SM, Weitz JI (2005) Coagulation assays. Circulation 112:e53–e60. doi:10.1161/CIRCULATIONAHA.104.478222
Gross PL, Weitz JI (2008) New anticoagulants for treatment of venous thromboembolism. Arterioscler Thromb Vasc Biol 28:380–386. doi:10.1161/ATVBAHA.108.162677
Rau JC, Beaulieu LM, Huntington JA et al (2007) Serpins in thrombosis, hemostasis and fibrinolysis. J Thromb Haemost 5:102–115. doi:10.1111/j.1538-7836.2007.02516.x
Jin L, Abrahams JP, Skinner R et al (1997) The anticoagulant activation of antithrombin by heparin. Proc Natl Acad Sci USA 94:14683–14688. doi:10.1073/pnas.94.26.14683
Shriver Z, Sundaram M, Venkataraman G et al (2000) Cleavage of the antithrombin III binding site in heparin by heparinases and its implication in the generation of low molecular weight heparin. Proc Natl Acad Sci USA 97:10365–10370. doi:10.1073/pnas.97.19.10365
Petitou M, Imberty A, Duchaussoy P et al (2001) Experimental proof for the structure of a thrombin-inhibiting heparin molecule. Chemistry (Easton) 7:858–873
Sundaram M, Qi Y, Shriver Z et al (2003) Rational design of low-molecular weight heparins with improved in vivo activity. Proc Natl Acad Sci USA 100:651–656. doi:10.1073/pnas.252643299
Naoum JJ, Woodside KJ, Zhang S et al (2005) Effects of rapamycin on the arterial inflammatory response in atherosclerotic plaques in Apo-E knockout mice. Transplant Proc 37:1880–1884. doi:10.1016/j.transproceed.2005.02.080
Naoum JJ, Zhang S, Woodside KJ et al (2004) Aortic eNOS expression and phosphorylation in Apo-E knockout mice: differing effects of rapamycin and simvastatin. Surgery 136:323–328. doi:10.1016/j.surg.2004.05.007
Wilson KM, Lynch CM, Faraci FM et al (2003) Effect of mechanical ventilation on carotid artery thrombosis induced by photochemical injury in mice. J Thromb Haemost 1:2669–2674. doi:10.1111/j.1538-7836.2003.00482.x
Vanichakarn P, Blair P, Wu C et al (2008) Neutrophil CD40 enhances platelet-mediated inflammation. Thromb Res 122:346–358. doi:10.1016/j.thromres.2007.12.019
Chakrabarti S, Varghese S, Vitseva O et al (2005) CD40 ligand influences platelet release of reactive oxygen intermediates. Arterioscler Thromb Vasc Biol 25:2428–2434. doi:10.1161/01.ATV.0000184765.59207.f3
Smyth SS, Reis ED, Vaananen H et al (2001) Variable protection of beta 3-integrin-deficient mice from thrombosis initiated by different mechanisms. Blood 98:1055–1062. doi:10.1182/blood.V98.4.1055
Severin S, Gratacap MP, Lenain N et al (2007) Deficiency of Src homology 2 domain-containing inositol 5-phosphatase 1 affects platelet responses and thrombus growth. J Clin Invest 117:944–952. doi:10.1172/JCI29967
Brey EM, Lalani Z, Johnston C et al (2003) Automated selection of DAB-labeled tissue for immunohistochemical quantification. J Histochem Cytochem 51:575–584
Furman MI, Krueger LA, Linden MD et al (2005) GPIIb-IIIa antagonists reduce thromboinflammatory processes in patients with acute coronary syndromes undergoing percutaneous coronary intervention. J Thromb Haemost 3:312–320. doi:10.1111/j.1538-7836.2005.01124.x
Furman MI, Kereiakes DJ, Krueger LA et al (2001) Leukocyte-platelet aggregation, platelet surface P-selectin, and platelet surface glycoprotein IIIa after percutaneous coronary intervention: effects of dalteparin or unfractionated heparin in combination with abciximab. Am Heart J 142:790–798. doi:10.1067/mhj.2001.119128
Furman MI, Barnard MR, Krueger LA et al (2001) Circulating monocyte-platelet aggregates are an early marker of acute myocardial infarction. J Am Coll Cardiol 38:1002–1006. doi:10.1016/S0735-1097(01)01485-1
Furman MI, Benoit SE, Barnard MR et al (1998) Increased platelet reactivity and circulating monocyte-platelet aggregates in patients with stable coronary artery disease. J Am Coll Cardiol 31:352–358. doi:10.1016/S0735-1097(97)00510-X
Westrick RJ, Eitzman DT (2007) Plasminogen activator inhibitor-1 in vascular thrombosis. Curr Drug Targets 8:966–1002. doi:10.2174/138945007781662328
Bates SM, Weitz JI (2000) The mechanism of action of thrombin inhibitors. J Invasive Cardiol 12:27–32
Gallus AS (2003) Preventing venous thromboembolism in general medical inpatients and after an ischaemic stroke. Haemostasis 30:64–71. doi:10.1159/000054166
Michelson AD, Barnard MR, Krueger LA et al (2001) Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation 104:1533–1537. doi:10.1161/hc3801.095588
Acknowledgements
These studies were partly supported by a grant from Momenta Pharmaceuticals, Cambridge, MA (JEF). Analysis of plasma samples was performed in a blinded manner by Brian Vozzella and Alison Long (anti-Xa, -IIa), at Momenta Pharmaceuticals, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chakrabarti, S., Beaulieu, L.M., Reyelt, L.A. et al. M118, a novel low-molecular weight heparin with decreased polydispersity leads to enhanced anticoagulant activity and thrombotic occlusion in ApoE knockout mice. J Thromb Thrombolysis 28, 394–400 (2009). https://doi.org/10.1007/s11239-009-0340-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-009-0340-4