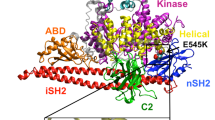
Abstract
ERK1 is an important kinase in Ras–Raf–MEK signaling. We have recently demonstrated by mass spectrometry that Tyr210 of ERK1 can be nitrated, and the nitration leads to a novel CHIP–dependent ERK1 degradation pathway. This motivated us to investigate the effect of this nitration on local and global structures of human ERK1 by Gaussian calculations and molecular dynamics simulations. Our study shows that the nitration at either the CE1 or the CE2 positions introduces an intra-hydrogen bond between the hydroxyl group and the nitro group of the nitrated Tyr210 of ERK1. Our data indicate that the hydrogen bond between Tyr210 and Glu237 is not stable and the nitration of Tyr210 facilitates its breakage. The previous studies demonstrated that ERK1 can be autophosphorylated at tyrosine residue 204. We next studied the effect of the phosphorylation in combination with the nitration. We found that the nitration of Tyr210 modestly decreases its inter-residue electrostatic interactions, and the phosphorylation of Tyr204 increases its inter-residue electrostatic interactions as expected. In the active state of ERK1, the conserved Lys71 forms a salt bridge with the conserved Glu88 and this salt bridge is essential for the activity of ERK1. Our data demonstrate an additional salt bridge for Glu88 with Arg84, and this salt bridge is unphosphorylation-dependent. The phosphorylation of ERK1 with the nitration at CE2, not at CE1, slightly enhances the K71–E88 salt bridge. Collectively, our simulations have demonstrated formation of a tyrosine nitration–dependent hydrogen bond. Our molecular dynamics simulations in combination with the experimental data on the nitration of Tyr210 of ERK1 indicate that a post-translational modification of a protein can specifically alter local structural environments, and a local structural change could lead to changes of protein functions.







Similar content being viewed by others
References
Lewis TS, Shapiro PS, Ahn NG (1998) Signal transduction through MAP Kinase Cascades. In: FVW G, George K (eds) Advances in cancer research, vol 74. Academic Press, pp 49–139
Buscà R, Pouysségur J, Lenormand P (2016) ERK1 and ERK2 map kinases: specific roles or functional redundancy? Front Cell Dev Biol 4(53)
Kinoshita T, Yoshida I, Nakae S, Okita K, Gouda M, Matsubara M, Yokota K, Ishiguro H, Tada T (2008) Crystal structure of human mono–phosphorylated ERK1 at Tyr204. Biochem Biophys Res Commun 377(4):1123–1127
Pagès G, Guérin S, Grall D, Bonino F, Smith A, Anjuere F, Auberger P, Pouysségur J (1999) Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice. Science 286(5443):1374–1377
Yao Y, Li W, Wu J, Germann UA, Su MSS, Kuida K, Boucher DM (2003) Extracellular signal–regulated kinase 2 is necessary for mesoderm differentiation. Proc Natl Acad Sci 100(22):12759–12764
Seger R, Ahn NG, Boulton TG, Yancopoulos GD, Panayotatos N, Radziejewska E, Ericsson L, Bratlien RL, Cobb MH, Krebs EG (1991) Microtubule–associated protein 2 kinases, ERK1 and ERK2, undergo autophosphorylation on both tyrosine and threonine residues: implications for their mechanism of activation. Proc Natl Acad Sci 88(14):6142–6146
Panta GR, Du L, Nwariaku FE, Kim LT (2005) Direct phosphorylation of proliferative and survival pathway proteins by RET. Surgery 138(2):269–274
Petersson GA, Bennett A, Tensfeldt TG, Al-Laham MA, Shirley WA, Mantzaris J (1988) A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row elements. J Chem Phys 89(4):2193–2218
Petersson GA, Al-Laham MA (1991) A complete basis set model chemistry. II. Open-shell systems and the total energies of the first-row atoms. J Chem Phys 94(9):6081–6090
Singh UC, Kollman PA (1984) An approach to computing electrostatic charges for molecules. J Comput Chem 5(2):129–145
Besler BH, Merz KM, Kollman PA (1990) Atomic charges derived from semiempirical methods. J Comput Chem 11(4):431–439
Wang J, Wang W, Kollmann P, Case D (2005) Antechamber, an accessory software PackageFor molecular mechanical calculation. J Comput Chem 25:1157–1174
Case DA, Cheatham 3rd TE, Darden T, Gohlke H, Luo R, Merz Jr KM, Onufriev A, Simmerling C, Wang B, Woods RJ (2005) The Amber biomolecular simulation programs. J Comput Chem 26(16):1668–1688
Homeyer N, Horn AHC, Lanig H, Sticht H (2006) AMBER force–field parameters for phosphorylated amino acids in different protonation states: phosphoserine, phosphothreonine, phosphotyrosine, and phosphohistidine. J Mol Model 12(3):281–289
Steinbrecher T, Latzer J, Case DA (2012) Revised AMBER parameters for bioorganic phosphates. J Chem Theory Comput 8(11):4405–4412
Simmerling C, Strockbine B, Roitberg AE (2002) All–atom structure prediction and folding simulations of a stable protein. J Am Chem Soc 124(38):11258–11259
Roe DR, Cheatham TE (2013) PTRAJ and CPPTRAJ: software for processing and analysis of molecular dynamics trajectory data. J Chem Theory Comput 9(7):3084–3095
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14(1):33–38 27–38
Onufriev A, Bashford D, Case DA (2004) Exploring protein native states and large–scale conformational changes with a modified generalized born model. Proteins 55(2):383–394
Roskoski Jr R (2012) ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res 66(2):105–143
Ferrell JE, Bhatt RR (1997) Mechanistic studies of the dual phosphorylation of mitogen–activated protein kinase. J Biol Chem 272(30):19008–19016
Taylor SS, Kornev AP (2011) Protein kinases: evolution of dynamic regulatory proteins. Trends Biochem Sci 36(2):65–77
Zhang F, Strand A, Robbins D, Cobb MH, Goldsmith EJ (1994) Atomic structure of the MAP kinase ERK2 at 2.3 a resolution. Nature 367(6465):704–711
Canagarajah BJ, Khokhlatchev A, Cobb MH, Goldsmith EJ (1997) Activation mechanism of the MAP kinase ERK2 by dual phosphorylation. Cell 90(5):859–869
Radi R (2013) Protein tyrosine nitration: biochemical mechanisms and structural basis of functional effects. Acc Chem Res 46(2):550–559
Moreno DM, Martí MA, De Biase PM, Estrin DA, Demicheli V, Radi R, Boechi L (2011) Exploring the molecular basis of human manganese superoxide dismutase inactivation mediated by tyrosine 34 nitration. Arch Biochem Biophys 507(2):304–309
Quint P, Reutzel R, Mikulski R, McKenna R, Silverman DN (2006) Crystal structure of nitrated human manganese superoxide dismutase: mechanism of inactivation. Free Radic Biol Med 40(3):453–458
Abriata LA, Cassina A, Tórtora V, Marín M, Souza JM, Castro L, Vila AJ, Radi R (2009) Nitration of solvent–exposed tyrosine 74 on cytochrome c triggers heme iron–methionine 80 bond disruption: nuclear magnetic resonance and optical spectroscopy studies. J Biol Chem 284(1):17–26
Godoy LC, Muñoz-Pinedo C, Castro L, Cardaci S, Schonhoff CM, King M, Tórtora V, Marín M, Miao Q, Jiang JF et al (2009) Disruption of the M80–Fe ligation stimulates the translocation of cytochrome c to the cytoplasm and nucleus in nonapoptotic cells. Proc Natl Acad Sci 106(8):2653–2658
Lai S, Pelech S (2016) Regulatory roles of conserved phosphorylation sites in the activation T–loop of the MAP kinase ERK1. Mol Biol Cell
Taylor SS, Radzio-Andzelm E (1994) Three protein kinase structures define a common motif. Structure 2(5):345–355
Johnson LN, Noble MEM, Owen DJ (1996) Active and inactive protein kinases: structural basis for regulation. Cell 85(2):149–158
Kornev AP, Haste NM, Taylor SS, Ten Eyck LF (2006) Surface comparison of active and inactive protein kinases identifies a conserved activation mechanism. Proc Natl Acad Sci 103(47):17783–17788
Acknowledgments
The authors thank the comments from Dr. Perkins on this study. The authors also thank the support from University Undergraduate Research Mini-Grant and STEP Awards and the equipment [LEQSF(2015-16)-ENH-TR-34] and software [LEQSF(2017-18)-ENH-TR-31] support from the Enhancement Program of Louisiana Board of Regents. The molecular dynamics simulations and Gaussian calculations of human ERK1 were conducted with high-performance computational resources provided by the Louisiana Optical Network Initiative (LONI) (http://www.loni.org).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PPTX 2343 kb)
Rights and permissions
About this article
Cite this article
Xu, W., Zhang, Y., Achi, O.Y. et al. Tyrosine nitration of human ERK1 introduces an intra-hydrogen bond by molecular dynamics simulations. Struct Chem 30, 1459–1470 (2019). https://doi.org/10.1007/s11224-019-01306-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-019-01306-z