Abstract
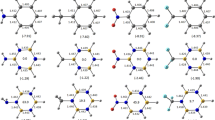
Geometrical parameters, aromaticity, and conformational flexibility of the set of polysubstituted benzenes with different number and position of nitro and amino groups were calculated at the MP2/cc-pvdz level of theory. The key factor for structural and energetic changes has been identified. This is related to the presence of nitro and amino groups in vicinal positions that forms strong intramolecular resonance-assisted hydrogen bonds with a binding energy of 7–14 kcal/mol. Increasing number of such bonds facilitates a cooperative effect, inducing notable changes in molecular geometry (particularly increasing bond alternation within H2N–C–C–NO2 fragment and planarization of amino group), drastic increasing of conformational flexibility and decreasing of aromaticity. In spite of well-known π-electron effects of nitro and amino substituents, influence of their push–pull interaction through aromatic moiety is negligible compared to the effect of the hydrogen bonding. That results in great difference of the ortho-isomers as compared to meta- and para-isomers.



Similar content being viewed by others
References
Krygowski TM, Cyranski MK (2001) Chem Rev 101:1385–1420
Schleyer PvR (2001) Chem Rev 101:1115–1117
Stock LM, Brown HC (1963) Adv Phys Org Chem 1:36–154
Hoggett JG, Moodie RB, Penton RB, Schofield K (1971) Nitration and aromatic reactivity. Cambridge University Press, Cambridge
Exner O, Bohm S (2002) J Org Chem 67:6320–6327
Krygowski TM, Stepien BT (2005) Chem Rev 105:3482–3512
Krygowski TM, Esmont K, Stepien BT, Cyranski MK, Poater J, Sola M (2004) J Org Chem 69:6634–6640
Shishkin OV, Omelchenko IV, Krasovska MV, Zubatyuk RI, Gorb L, Leszczynski J (2006) J Mol Struct 791:158–164
Krygowski TM, Stepien BT, Cyranski MK (2005) Int J Mol Sci 6:45–51
Smith MB, March J (2001) Advanced organic chemistry. Wiley, New York
Krygowski TM, Dobrowolski MA, Zborowski K, Cyranski MK (2006) J Phys Org Chem 19:889–895
Roszak S, Gee RH, Balasubramanian K, Fried LE (2003) Chem Phys Lett 374:286
Lima CFRAC, Gomes LR, Santos LMNBF (2007) J Phys Chem A 111:10598–10603
Krygowski TM, Palusiak M, Plonka A, Zachara-Horeglad JEJ (2007) Phys Org Chem 20:297–306
Zhang C (2006) Chem Phys 324:547–555
Szatylowicz H, Krygowski TM, Hobza P (2007) J Phys Chem A 111:170–175
Borisenko VE, Krekov SA, MYu Fomenko, Koll A, Lipkovski PJ (2008) Mol Struct 882:9–23
Gross KC, Seybold PG, Peralta-Inga Z, Murray JS, Politzer P (2001) J Org Chem 66:6919–6925
Alonso M, Herradon B (2010) Phys Chem Chem Phys 12:1305–1317
Chermahini AN, Dabbagh HA, Teimouri A (2007) J Mol Struct 822:33–37
Badgujar DM, Talawar MB, Asthana SN, Mahulikar PP (2008) J Hazard Mater 151:289–305
Keshavarz MH (2008) J Hazard Mater A 153:201–206
Takemura N, Shimizu H (1978) Mutat Res 54:256–257
Luther M (1990) Chemosphere 21:231–241
Levine BF (1976) Chem Phys Lett 37:516–520
Wolleben J, Testa AC (1977) J Phys Chem 81:429–431
in het Panhuis M, Munn RW, Popelier PLA (2004) J Chem Phys 120:11479–11486
Wang JX, Gong XD, Xiao HM (2009) Int J Quant Chem 109:1522–1530
Manaa MR, Gee RH, Fried LE (2002) J Phys Chem A 106:8806–8810
Gee RH, Roszak S, Balasubramanian K, Fried LE (2004) J Chem Phys 120:7059–7066
Møller C, Plesset MS (1934) Phys Rev 46:618–622
Kendall RA, Dunning TH Jr, Harrison RJ (1992) J Chem Phys 96:6796–6806
Minkin VI, Glukhovtsev MN, Simkin BY (1994) Aromaticity and antiaromaticity. Wiley, New York
Cyranski MK (2005) Chem Rev 105:3773–3811
Cyranski MK, Krygowski TM, Katritzky AR, Schleyer PvR (2002) J Org Chem 67:1333–1338
Jug K, Oniciu DC, Katritzky AR (2001) Chem Rev 101:1421–1449
Alonso M, Herradon BJ (2010) Comp Chem 31:917–928
Poater J, Duran M, Sola M, Silvi B (2005) Chem Rev 105:3911–3947
Bird CW (1992) Tetrahedron 48:335–340
Cyranski MK, Krygowski TM (1999) Tetrahedron 55:6205–6210
Chen Z, Wannere CS, Corminboeuf C, Putcha R, Schleyer PvR (2005) Chem Rev 105:3842–3888
Shishkin OV, KYu Pichugin, Gorb L, Leszczynski J (2002) J Mol Struct 616:159–166
Zhigalko MV, Shishkin OV, Gorb L, Leszczynski J (2004) J Mol Struct 693:153–159
Shishkin OV, Gorb L, Lesczynski J (2000) Chem Phys Lett 330:603–611
Gordy WJ (1947) J Chem Phys 15:305–310
Fallah-Bagher-Shaidaei H, Wannere CS, Corminboeuf C, Puchta R, Schleyer PvR (2006) Org Lett 8:863–866
Gaussian 03, Revision C.01, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, and Pople JA (2004) Gaussian, Inc., Wallingford CT
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347–1363
Bader RWF (1990) Atoms in molecules. A quantum theory. Calendon Press, Oxford
Espinosa E, Molina E, Lecomte C (1998) Chem Phys Lett 285:170–173
Dorofeeva OV, Vishnevskiy YV, Vogt N, Vogt J, Khristenko LV, Krasnoshchekov SV, Shishkov IF, Hargittai I, Vilkov LV (2007) Struct Chem 18:739–753
Schultz G, Portalone G, Ramondo F, Domenicano A, Hargittai I (1996) Struct Chem 7:59–71
Sinclair WE, Pratt DW (1996) J Chem Phys 105:7942–7956
Colapietro M, Domenicano A, Portalone G, Schultz G, Hargittai I (1987) J Phys Chem 91:1728–1737
Sadova NI, Penionzhkevich NP, Vilkov LV (1976) J Struct Chem (in Russian) 17:954–956
Zych T, Misiaszek T, Szostak MM (2007) Chem Phys 340:260–272
Colapietro M, Domenicano A, Marciante C, Portalone G (1982) Z Naturforsch 37B:1309–1311
Qian HY, Yin ZG, Jia J, Zhou N, Feng LQ (2006) Acta Crystallogr 62E:o5048–o5049
Wojcik G, Holband J (2001) Acta Crystallogr 57B:346–352
Woodford JN, Pauley MA, Wang CH (1997) J Phys Chem 101B:1989–1992
Pappalardo RR, Marcos ES, Ruiz-López MF, Rinaldi D, Rivail JL (1993) J Am Chem Soc 115:3722–3730
Kovacs A, Szabo A, Hargittai I (2002) Acc Chem Res 35:887–894
Chung G, Kwon O, Kwon Y (1997) J Phys Chem A 101:4628–4632
Borisenko KB, Zauer K, Hargittai I (1996) J Phys Chem 100:19303–19309
Gilli G, Belucci F, Ferretti V, Bertolesi V (1989) J Am Chem Soc 111:1023–1028
Sobczyk L, Grabowski SJ, Krygowski TM (2005) Chem Rev 105:3513–3560
Manaa MR, Fried LE (2001) J Phys Chem A 105:6765–6768
Rashid AN (2004) J Mol Struct 681:57–63
Cohen AJ, Mori-Sanchez P, Yang W (2008) Science 321:792–794
Allen FH (2002) Acta Cryst B58:380–388
Fazli M, Raissi H, Chahkandi B, Aarabi M (2010) J Mol Struct 942:115–120
Wojtulewski S, Grabowski SJ (2003) J Mol Struct 621:285–291
Huanga Z, Chenb B, Gao G (2005) J Mol Struct 752:87–92
Kimmel AV, Sushko PV, Shluger AL, Kuklja MM (2008) J Phys Chem A 112:4496–4500
Liu H, Zhao J, Ji G, Wei D, Gong Z (2006) Phys Lett A 358:63–69
Pravica M, Yulga B, Tkachev S, Liu Z (2009) J Phys Chem A 113:9133–9137
Wu C, Fried LE (2000) J Phys Chem A 104:6447–6452
Dobratz BM (1995) The insensitive high explosive triaminotrinitrobenzene (TATB): development and characterizations—1888 to 1994. Los Alamos National Laboratory, Los Alamos
Kotelevskii SI, Prezhdo OV (2001) Tetrahedron 57:5715–5729
Acknowledgments
The use of trade, product, or firm names in this report is for descriptive purposes only and does not imply endorsement by the U.S. Government. Results in this study were funded and obtained from research conducted under the Environmental Quality Technology Program of the United States Army Corps of Engineers by the USAERDC. Permission was granted by the Chief of Engineers to publish this information. The findings of this report are not to be construed as an official Department of the Army position unless so designated by other authorized documents.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Omelchenko, I.V., Shishkin, O.V., Gorb, L. et al. Properties, aromaticity, and substituents effects in poly nitro- and amino-substituted benzenes. Struct Chem 23, 1585–1597 (2012). https://doi.org/10.1007/s11224-012-9971-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-012-9971-8