Abstract
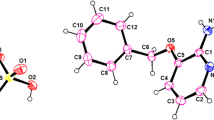
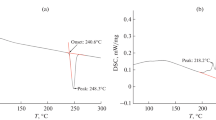
Mixed salts (NH4)2SO4·2NH4NO3 (1) and (NH4)2SO4·3NH4NO3 (2) were synthesized and studied by X-ray diffraction analysis. The unit cell parameters of these salts were determined and their crystal structures were solved. The thermal stability of the salts was studied by differential scanning calorimetry and thermogravimetric analysis. The temperatures and enthalpies of incongruent melting of compounds 1 and 2 were determined. The enthalpies of formation from the constituent salts were estimated.
Similar content being viewed by others
References
M. A. Miniovich, Soli azotnoi kisloty [Salts of Nitric Acid], Izd. Khim. Literatury, Moscow, 1946, 192 pp. (in Russian).
E. Janecke, W. Eisskee, R. Brigl, Z. anorg. allg. Chem., 1927, 160, 171.
V. A. Sokolov, Izv. AN SSSR. Otd. Estestv. Matem. Nauk [Izv. Akad. Nauk USSR, Ser. Natur. Mathem. Sci.], 1938, 124 (in Russian).
R. V. Coates, G. D. Woodard, J. Sci. Food Agric., 1963, 14, 398.
J. P. Smith, J. R. Lehr, A. W. Frazier, J. Agric. Food Chem., 1962, 10, 77.
E. D. Perman, W. J. Howells, F. Chem. Soc., Trans., 1923, 2128.
H. Kiiski, Dis. Ph. D., University of Helsinky, Faculty of Science Department of Chemistry, Helsinky, 2009, 238 pp.; http://www.doria.fi/bitstream/handle/10024/47167/properti.pdf.
I. N. Nikonova, A. G. Bergman, Zh. Prikl. Khim., 1942, 15, 437 [J. Appl. Chem. (Engl. Transl.), 1942, 15].
J. A. Kweeder, N. E. Iwamoto, Detonation-Resistant Fertiliser Composition Comprising Ammonium Nitrate Double Salts, Pat. US 0199357 Al. 2007; Chem. Abstrs, 2007, 147, 188472.
M. Nagatani, T. Seiyama, Bull. Chem. Soc. Jpn, 1967, 40, 1833.
L. V. Gurvich, I. V. Veits, V. A. Medvedev, G. A. Khachkuruzov, V. S. Yungman, Termodinamicheskie svoistva individual’nykh veshchestv [Thermodynamic Properties of Individual Substances], Ed. V. P. Glushko, Nauka, Moscow, 1978, p. 344 (in Russian).
G. M. Sheldrick, Acta Crystallogr., Sect. A, 2008, 64, 112.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 1, pp. 33—38, January, 2012.
Rights and permissions
About this article
Cite this article
Babkina, T.S., Golovina, N.B., Bogachev, A.G. et al. Crystal structures and physicochemical properties of mixed salts of ammonium nitrate and sulfate. Russ Chem Bull 61, 33–39 (2012). https://doi.org/10.1007/s11172-012-0005-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-012-0005-x