Abstract
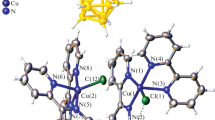
The newly synthesized complex (2) of copper(I) chloride with di-n-hexyl 2,2′-biquinoline-4,4′-dicarboxylate (L) was spectroscopically and electrochemically characterized. The X-ray diffraction study showed that the crystals of complex 2 consist of the dinuclear moieties [L2Cu1 2(μ-Cl)2] containing Cu2(μ-Cl)2 clusters. Spectrophotometric studies and ESI-mass spec-trometric measurements showed that after the dissolution of complex 2 in acetonitrile (AN) and N-methyl-2-pyrrolidone (NMP), the solution contained not only the dinuclear complexes [L2Cu1 2(μ-Cl)2] but also [L2Cu1]Cl, [LCu1Cl(Sol)], and [Cu1Cl(Sol)] (Sol is the solvent). The electrochemical data also confirm the conclusion that bridged dinuclear chloride complex 2 dissociates both in NMP and AN to form the tetrahedral bis-biquinoline complex [L2Cu1]Cl. In solutions of complex 2 in alcohols and N,N-dimethylformamide (DMF), only [L2Cu1]Cl and [Cu1Cl(Sol)] are present. In EtOH, AN, and DMF, [Cu1Cl(Sol)] undergoes disproportionation to [Cu11Cl(Sol)] and Cu0.
Similar content being viewed by others
References
Comprehensive Coordination Chemistry II: From Biology to Nanotechnology, Eds J. A. McCleverty, T. J. Meyer, Vol. 1, Elsevier, Amsterdam, 2005.
F. Durola, J.-P. Savage, Angew. Chem., Int. Ed., 2007, 46, 3537.
V. Balzani, A. Credi, S. Silvi, M. Venturi, Chem. Soc. Rev., 2006, 35, 1135.
S. Kawano, N. Fujita, S. Shinkai, J. Am. Chem. Soc., 2004, 126, 8592.
J. V. Steed and J. L. Etwood, Supramolecular Chemistry, John Wiley and Sons, Ltd, Chichester-New York—Weinheim—Brisbane—Singapore—Toronto, 2000.
V. V. Skopenko, A. Yu. Tsivadze, L. I. Savranskii, A. D. Garnovskii, Koordinatsionnaya khimiya [Coordination Chemistry], Akademkniga, Moscow, 2007, 487 p. (in Russian).
T. Nabeshima, T. Inaba, N. Furukawa, Inorg. Chem., 1993, 32, 1407.
S. M. Scott, K. C. Gordon, Inorg. Chem., 1996, 35, 2452.
Y. Jahng, J. Hazelrigg, D. Kimball, E. Riesgo, F. Wu, R. P. Thummel, Inorg. Chem., 1997, 36, 5390.
M. D. Stephenson, M. J. Hardie, Dalton Trans., 2006, 3407.
L. Carlucci, G. Ciani, A. Gramaccioli, D. M. Proserpio, S. Rizzato, Cryst. Eng. Commun., 2000, 1.
O. Gonzalez, A. M. Atria, E. Spodine, J. Manzur, M. T. Garland, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1993, 49, 1589.
E. Kovari, R. Kramer, Z. Naturforsch., B: Chem. Sci., 1994, 49, 1324.
A. Alvarez-Larena, J. L. Brianso-Penalva, J. F. Piniella, R. Moreno-Esparza, L. Ruiz-Ramirez, A. Tovar, Z. Kristal-logr., 1995, 210, 543.
A. M. Atria, P. Cortes, L. Acevedo, R. Trujillo, J. Manzur, O. Pena, R. Baggio, J. Chil. Chem. Soc., 2004, 49, 341.
E. Tynan, P. Jensen, A. C. Lees, B. Moubaraki, K. S. Murray, P. E. Kruger, Cryst. Eng. Commun., 2005, 90.
G. E. Kostakis, E. Nordlander, M. Haukka, J. C. Plakatouras, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2006, 62, m77.
M. Ghosh, P. Biswas, U. Floerke, K. Nag, Inorg. Chem., 2008, 47, 281.
F. M. Menger, J.-J. Lee, K. S. Hagen, J. Am. Chem. Soc. 1991, 113, 4017.
A. M. Atria, R. F. Baggio, M. T. Garland, E. Spodine, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1994, 50, 864.
B. Viossat, J. F. Gaucher, A. Mazurier, M. Selkti, A. Tomas, Z. Kristallogr.-New Cryst. Struct., 1998, 213, 329.
Y.-Q. Zheng, J. Sun, J.-L. Lin, Z. Anorg. Allg. Chem., 2001, 627, 90.
J.-H. Yu, Z.-L. Lu, J.-Q. Xu, H.-Y. Bie, J. Lu, X. Zhang, New J. Chem., 2004, 28, 940.
L. Wang, R.-B. Huang, L.-S. Long, L.-S. Zheng, E.-B. Wang, Z.-X. Xie, J. Coord. Chem., 2005, 58, 1439.
Y. Muranishi, Y. Wang, M. Odoko, N. Okabe, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2005, 61, m307.
R. M. Williams, L. De Cola, F. Hartl, J. Lagref, J.-M. Planeix, A. De Cian, M. W. Hosseini, Coord. Chem. Rev., 2002, 230, 253.
S. Kitagawa, M. Munakata, N. Miyaji, Bull. Chem. Soc. Jpn, 1983, 56, 2258.
M. Munakata, M. Maekawa, S. Kitagawa, S. Nishibayashi, Kinki Daigaku Rikogakubu Kenkyu Hokoku, 1989, 25, 81; Chem.Abstrs, 1990, 112, 138441.
R. J. Butcher, E. Sinn, Inorg. Chem., 1977, 16, 2334.
E. Sinn, J. Chem. Soc., Dalton Trans., 1976, 162.
B. F. Ali, K. Al-Sou ’od, N. Al-Ja ’ar, A. Nassar, M. H. Zaghal, Z. Judeh, R. Al-Far, M. Al-Refai, M. Ibrahim, K. Mansi, K. H. Al-Obaidi, J. Coord. Chem., 2006, 59, 229.
A. L. Gershuns, A. A. Verezubova, Zh. A. Tolstykh, Izv. Vuzov, Ser. Khim. i Khimich. Tekh. [Proceedings of Institutes: Chemistry and Chemical Technology], 1961, No. 1, 25.
H. R. Al-Obaidi, K. C. Gordon, J. J. McGarvey, S. E. J. Bell, J. Grimshaw, J. Phys. Chem., 1993, 97, 10942.
R. Bilewicz, M. Pietraszkiewicz, Polyhedron, 1990, 9, 2353.
T. V. Magdesieva, A. V. Dolganov, A. V. Yakimanskii, M. Ya. Goikhman, I. V. Podeshvo, V. V. Kudryavtsev, Electrochemistry, 2007, 43, 1194 [Russ. J. Electrochem. (Engl. Transl.), 2007,43].
K. Stolarczyk, R. Bilewicz, L. Siegfried, T. Kaden, Inorg. Chem. Acta, 2003, 348, 129.
T. V. Magdesieva, A. V. Dolganov, P. M. Poleshchuk, A. V. Yakimanskii, M. Ya. Goikhman, I. V. Podeshvo, V. V. Kudryavtsev, Izv. Akad. Nauk, Ser. Khim., 2007, 1331 [Russ. Chem. Bull., Int. Ed., 2007, 56, 1380].
S. D. Lesesne, H. R. Henze, J. Am. Chem. Soc., 1942, 64, 1897.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 4, pp. 710–718, April, 2010
Rights and permissions
About this article
Cite this article
Vatsadze, S.Z., Dolganov, A.V., Yakimanskii, A.V. et al. Spectroscopic and electrochemical study of dinuclear and mononuclear copper complexes with the bidentate ligand of the 2,2′-diquinoline series. Russ Chem Bull 59, 724–732 (2010). https://doi.org/10.1007/s11172-010-0153-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-010-0153-9